Synthesis of new chiral lactam-fused pyridine derivatives†
Abstract
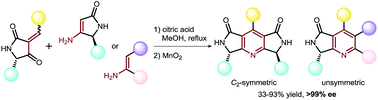
New chiral C2-symmetric and unsymmetric lactam-fused pyridines have been synthesised in enantiomerically pure forms with satisfactory yields via an acid-promoted cyclisation of benzylidene-modified tetramic acids and various enamines.

 Please wait while we load your content...
Please wait while we load your content...