Photoresponsive and wetting performances of sheet-like nanostructures of tungsten trioxide thin films grown on wood surfaces
Abstract
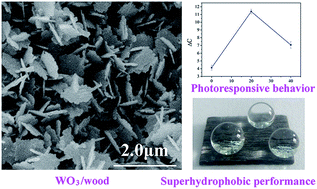
Tungsten trioxide films with sheet-like nanostructures were grown on wood surfaces through a simple low-temperature hydrothermal method using ethanol as an inducer. The morphologies, phase structures, compositions, optical properties and wetting behaviors of the WO3-treated wood surfaces were characterized. The ethanol content in the precursor solution played an important role in the morphologies and phase structures. It was observed that the WO3 film based on the wood substrate was composed of 2D nanosheets with an edge length of 580–957 nm and a thickness of about 80 nm and was highly crystallized with a phase-pureness. The UV-vis analysis shows that the sample prepared with the 20% ethanol content responded to ultraviolet radiation of wavelength 270–350 nm very intensively. When the sample was irradiated under UV light (365 nm), there appeared an obvious color difference value (ΔC). The water contact angle (WCA) measurements demonstrated that the WO3-treated wood surfaces possessed superhydrophilic behavior and then converted to the superhydrophobic property through the octadecyltrichlorosilane (OTS) treatment. The AFM analysis indicated that a change in surface roughness of the film was responsible for photochromic and hydrophobic performances. The obtained wood surfaces exhibited photoresponsive and self-cleaning functions, which have potential applications such as information storage, smart sensors, and anti-fake materials.

 Please wait while we load your content...
Please wait while we load your content...