Improved mechanical properties of polyacrylamide hydrogels created in the presence of low-molecular-weight hydrogelators†
Yutaka Ohsedo*a,
Makiko Taniguchia,
Kowichiro Saruhashib and
Hisayuki Watanabeab
aAdvanced Materials Research Laboratory, Collaborative Research Division, Art, Science and Technology Center for Cooperative Research, Kyushu University, 4-1 Kyudaishinmachi, Nishi-ku, Fukuoka 819-0388, Japan. E-mail: ohsedo@astec.kyushu-u.ac.jp; Fax: +81-92-400-4382; Tel: +81-92-400-4381
bNissan Chemical Industries, Ltd., 2-10-1 Tsuboinishi, Funabashi, Chiba 274-8507, Japan
First published on 16th October 2015
Abstract
Polyacrylamide hydrogels were prepared using molecular hydrogels composed of a low-molecular-weight gelator system, octylaldonamide/sodium dodecyl sulfate as templates by photopolymerization, and the effect of the template on the polymer hydrogel network was examined on the basis of the polymer hydrogels' performance in compression tests. It was found that the crushing stress of the obtained polymer hydrogels was enhanced by using the molecular gel templates.
The synthesis, properties and applications of molecular gels composed of low-molecular-weight gelators (LMWGs) have received much attention.1,2 The unique properties of molecular gels make them attractive as functional soft matter for many applications.3,4 Compared to polymer gels,5 molecular gels have greater flexibility in their design and a higher probability for one-to-one correspondence in their structure–property relationships due to their defined chemical structures (polymer materials inevitably have dispersity in their molecular weights).
To enhance the properties of molecular gels, mixing of well-designed LMWGs has been widely investigated.6 Previously,7 we simply mixed LMWGs with different alkyl chains to alter the quality of the network structure constructed in molecular gels and demonstrated the mixing-induced thixotropy of several systems involving molecular hydrogels and organogels. Recently, an alternative strategy involving the mixing of LMWGs and polymer materials was vigorously examined, and the successful enhancement of molecular gel systems as new soft materials was achieved.8
Polymer gels generally have a level of inhomogeneity in their network structures, which are composed of polymer chains, as determined by diffraction analysis. This inhomogeneity is thought to contribute to the mechanical weakness of polymer gels because it inhibits the efficient dissipation of applied mechanical energy.9 Polymer gels have received much attention for their potential biomedical applications, but their mechanical properties require enhancement.5 Recently, several methods for removing the inhomogeneities in polymer networks and/or obtaining better network homogeneity have been reported. These methods offer new criteria for the preparation of polymer gels with homogeneous network structures which prevent stress concentration are advantageous for energy dissipation and thus have improved mechanical properties.10
We envisioned the use of LMWGs as templating materials and reaction vessels for the synthesis of polymer gels with improved properties. LMWGs are known to form gel-like materials composed of networks of crystalline fibres with sub-micrometre-order diameters rather than crystalline precipitates. We envisioned that the presence of an LMWG fibre network prepared via polymerisation and gelation may reduce the sub-micrometre-order inhomogeneity in polymer gel networks due to the reflection of the LMWG fibre network structure to some extent. We, therefore, synthesised polymer hydrogels in the presence of LMWG networks to investigate the effect of the LMWG on the mechanical properties of the prepared polymer hydrogels after removal of the LMWG. Herein, we describe the use of LMWGs as templating materials for the synthesis of polymer gels with reduced inhomogeneity. Whereas several researchers have reported the use of molecular gel fibres as fillers for polymers,8 there is no report on the use of a molecular gel as a removable template (Scheme 1).
 | ||
| Scheme 1 Schematic illustration of the concept of polymer gel creation with molecular gel template which is composed of LMWG. | ||
We selected cross-linked polyacrylamide (PAAm) as the polymer gel in this study. Acrylamide (AAm), methylenebisacrylamide (BIS) and 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (HEPP) were used as the monomer, cross-linker and photoinduced free radical initiator, respectively, for synthesis of the polymer hydrogel (Scheme 2). For the template, the molecular hydrogel system composed of the LMWG octylaldonamide (C8NG)11 with sodium dodecyl sulfate (SDS) was selected because it is known to form a stable molecular hydrogel consisting of a network of sub-micrometre-diameter fibres.11,12 First, a 2 M AAm aqueous solution containing C8NG at x wt% (x = 0.0, 3.0, 5.0 or 10.0), 0.5 wt% SDS, 0.02 M BIS and 2 mM HEPP was heated at 100 °C in the dark in order to dissolve the C8NG, and then at the solution as allowed to stand at room temperature until the C8NG/SDS molecular hydrogel was obtained. The vial inversion method was used to determine the gelation state of the mixture. Subsequently, the photoinitiated radical polymerisation of the translucent molecular hydrogel with AAm was performed by irradiating the solution with ultraviolet light (10 mW cm−2) for 5 min at 25 °C. Once gelation occurred, the mixture of polymer and molecular hydrogels was immersed and washed in hot water (70 °C) for 24 h to remove the C8NG, SDS and any unreacted AAm, BIS and HEPP. Removal of the initiator, cross-linker, monomer, C8NG and SDS was confirmed by elemental analysis of the washed and vacuum-dried hydrogels (Tables S1 and S2, see ESI†). The results of the elemental analyses confirmed that no other chemicals were present in the obtained hydrogels after washing, however, the presence of bound water was suggested. In addition, the swelling ratios of polymer hydrogels obtained using various concentrations of C8NG were nearly the same (q = 10–12), indicating that they possessed similar hydrogel networks. Contrary to our intention, however, whilst the obtained polymer hydrogel with no LMWG template was transparent, those prepared using the LMWG templates were slightly translucent (Fig. S1, ESI†). These results may suggest that the presence of the LMWG throughout the polymerisation induced some aggregation of the polymer chains, resulting in light scattering.
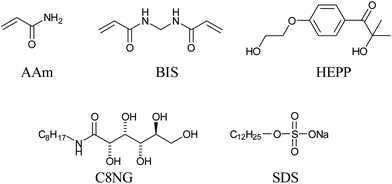 | ||
| Scheme 2 Chemical structures for polymer hydrogel (monomers and photo-induced radical initiator, upper row) and molecular gels (LMWG and surfactant, lower row). | ||
FT-IR spectra of the washed and vacuum-dried polymer hydrogels (xerogels) prepared using various concentration of C8NG and the polymer hydrogel synthesised without the molecular hydrogel template contained nearly the same peaks (Fig. S2, ESI†). In addition, 13C-NMR spectra of the solid state samples (xerogels) were obtained and also found to be nearly the same (Fig. S3, ESI†). These results indicate that the polymer hydrogels prepared using various C8NG concentrations contained similar chemical components.
To evaluate the effect of the presence of the molecular hydrogels throughout the gelation process on the mechanical properties of the polymer hydrogels, the crushing stress of the hydrogels was determined by performing compression tests. Each polymer hydrogel was cut and punched into cylindrical pieces (diameter: 8.0 mm; height: 5.0 mm). In the strain–stress curves for the polymer hydrogels (Fig. 1), it can be seen that the crushing stress, which is the compression stress at the inflection point of each curve, increased as the concentration of C8NG increased up to 5.0 wt% (Table 1, 0.08 MPa for 0.0 wt% C8NG, 0.12 MPa for 3.0 wt% C8NG and 0.16 MPa for 5.0 wt% C8NG), then decreased at 10.0 wt% C8NG (0.13 MPa). In addition, the fracture strain, which is the strain at the inflection point of the curve, also increased as the concentration of C8NG increased (Table 1, 52 mm mm−1 for 0.0 wt% C8NG, 57 mm mm−1 for 3.0 wt% C8NG, 67 mm mm−1 for 5.0 wt% C8NG and 68 mm mm−1 for 10.0 wt% C8NG). These results revealed that the crushing stress and fracture strain of the polymer hydrogels were enhanced by the presence of the molecular hydrogels as a function of the molecular gel concentration.
| Sample | qa | Crushing stressb (MPa) | Fracture strainc (mm mm−1) |
|---|---|---|---|
| a Degree of swelling based on weight (see ESI).b Compression stress at the inflection point of the curve.c Strain at the inflection point of the curve. | |||
| PAAm gel | 11 | — | — |
| PAAm gel (C8NG 0.0 wt%/SDS 0.5 wt%) | 12 | 0.08 | 52 |
| PAAm gel (C8NG 3.0 wt%/SDS 0.5 wt%) | 10 | 0.12 | 57 |
| PAAm gel (C8NG 5.0 wt%/SDS 0.5 wt%) | 12 | 0.16 | 67 |
| PAAm gel (C8NG 10.0 wt%/SDS 0.5 wt%) | 12 | 0.13 | 68 |
To investigate the microstructure of the molecular hydrogel systems, scanning electron microscopy (SEM) images of the C8NG/SDS xerogels were obtained. In Fig. S4 (ESI†), unfortunately, wrinkled tape-like structures with sub-micrometre thicknesses can be seen that were likely generated as the result of aggregation of fine sub-micrometre-diameter fibres composed of the LMWG during the freeze-drying process to form the xerogels. It appears that the sub-micrometre fibres of C8NG/SDS in the molecular hydrogels existed as network components. Notably, it can be seen in Fig. S4 (ESI†) that no remarkable thickening of the fibres occurred as the concentration of LMWG increased. Based on these results for the C8NG/SDS hydrogel system, it can be concluded that an increase in the concentration of the LMWG led to an increase in the fibre network density in the molecular gels. It is thought that up to 5 wt% C8NG, the presence of the molecular gel network template led to a reduction in some of the inhomogeneity in the polymer gel during the gelation process, likely due to the prevention of aggregation of the polymer chains. On the other hand, the decrease in the crushing stress of the polymer hydrogel prepared using the 10 wt% C8NG template is as yet unexplained. It is possible that the higher network density of the molecular gel may have promoted the deactivation of radical species during the polymerisation due to the presence of the OH groups in the C8NG.
In summary, a new method for the synthesis of polymer hydrogels with reduced inhomogeneity was developed by using a molecular gel as a removable template. The polymer hydrogels obtained using the LMWG template exhibited enhanced mechanical properties, such as increased crushing stresses. These improved properties may be due to more efficient energy dissipation made by the removal of inhomogeneity in the polymer network. These results will contribute to the development of new methods for the preparation of novel functional gels.
Notes and references
- For books: (a) Molecular Gels: Materials With Self-assembled Fibrillar Networks, ed. R. G. Weiss and P. Terech, Kluwer Academic Pub, 2006 Search PubMed; (b) Low Molecular Mass Gelators Design, Self-Assembly, Function, Top Curr. Chem., ed. F. Fages, Springer-Verlag Berlin Heidelberg, 2005, vol. 256 Search PubMed.
- For recent reviews: (a) A. Dawn, T. Shiraki, S. Haraguchi, S.-I. Tamaru and S. Shinkai, Chem.–Asian. J., 2011, 6, 266 CrossRef CAS PubMed; (b) K.-Q. Liu, P.-L. He and Y. Fang, Sci. China: Chem., 2011, 54, 575 CrossRef CAS; (c) J. W. Steed, Chem. Commun., 2011, 47, 1379 RSC; (d) X.-Y. Yang, G.-X. Zhang and D.-Q. Zhang, J. Mater. Chem., 2012, 22, 38 RSC; (e) A. J. Jonker, D. W. P. M. Löwik and J. C. M. van Hest, Chem. Mater., 2012, 24, 759 CrossRef CAS; (f) S. S. Babu, S. Prasanthkumar and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 1766 CrossRef CAS PubMed; (g) M. Yamanaka, J. Inclusion Phenom. Macrocyclic Chem., 2013, 77, 33 CrossRef CAS; (h) A. Y.-Y. Tam and V. W.-W. Yam, Chem. Soc. Rev., 2013, 42, 1540 RSC; (i) G.-C. Yu, X.-Z. Yan, C.-Y. Han and F.-H. Huang, Chem. Soc. Rev., 2013, 42, 6697 RSC; (j) Y. Feng, Y.-M. He and Q.-H. Fan, Chem.–Asian J., 2014, 9, 1724 CrossRef CAS PubMed; (k) S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed; (l) D. K. Kumar and J. W. Steed, Chem. Soc. Rev., 2014, 43, 2080 RSC; (m) X.-D. Yu, L.-M. Chen, M.-M. Zhang and T. Yi, Chem. Soc. Rev., 2014, 43, 5346 RSC; (n) R. G. Weiss, J. Am. Chem. Soc., 2014, 136, 7519 CrossRef CAS PubMed; (o) M. Cametti and Z. Džolić, Chem. Commun., 2014, 50, 8273 RSC; (p) M. A. Rogers and R. G. Weiss, New J. Chem., 2015, 39, 785 RSC; (q) J. Raeburn and D. J. Adams, Chem. Commun., 2015, 51, 5170 RSC.
- For medical application of LMWG: (a) A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002 CrossRef CAS PubMed; (b) F. Zhao, M. L. Ma and B. Xu, Chem. Soc. Rev., 2009, 38, 883 RSC; (c) J. B. Matson and S. I. Stupp, Chem. Commun., 2012, 48, 26 RSC; (d) J. Boekhoven and S. I. Stupp, Adv. Mater., 2014, 26, 1642 CrossRef CAS PubMed; (e) X. Du, J. Zhou and B. Xu, Chem.–Asian J., 2014, 9, 1446 CrossRef CAS PubMed; (f) A. Tanaka, Y. Fukuoka, Y. Morimoto, T. Honjo, D. Koda, M. Goto and T. Maruyama, J. Am. Chem. Soc., 2015, 137, 770 CrossRef CAS PubMed.
- For optoelectrical application of LMWG: (a) S. S. Babu, S. Prasanthkumar and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 1766 CrossRef CAS PubMed; (b) S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed.
- (a) Polymer Gels: Fundamentals and Biomedical Applications, ed. D. DiRossi, K. Kajiwara, Y. Osada and A. Yamauchi, Plenum Press, New York, 1991 Search PubMed; (b) Polymer Gels and Networks, ed. Y. Osada and A. R. Khokhlov, Marcel Dekker, New York, 2002 Search PubMed.
- For reviews of multi-component LMWG gels: (a) A. R. Hirst and D. K. Smith, Chem.–Eur. J., 2005, 11, 5496 CrossRef CAS PubMed; (b) C. A. Dreiss, Soft Matter, 2007, 3, 956 RSC; (c) L. E. Buerkle and S. J. Rowan, Chem. Soc. Rev., 2012, 41, 6089 RSC; (d) D. J. Cornwell and D. K. Smith, Mater. Horiz., 2015, 2, 279 RSC; (e) J. Raeburn and D. J. Adams, Chem. Commun., 2015, 51, 5170 RSC.
- (a) Y. Ohsedo, M. Miyamoto, H. Watanabe, M. Oono and A. Tanaka, Bull. Chem. Soc. Jpn., 2013, 86, 671 CrossRef CAS; (b) Y. Ohsedo, H. Watanabe, M. Oono and A. Tanaka, Chem. Lett., 2013, 42, 363 CrossRef CAS; (c) Y. Ohsedo, H. Watanabe, M. Oono and A. Tanaka, RSC Adv., 2013, 3, 5803 RSC; (d) Y. Ohsedo, M. Oono, A. Tanaka and H. Watanabe, New J. Chem., 2013, 37, 2250 RSC; (e) Y. Ohsedo, M. Taniguchi, M. Oono, K. Saruhashi and H. Watanabe, RSC Adv., 2014, 4, 35484 RSC; (f) Y. Ohsedo, M. Oono, K. Saruhashi and H. Watanabe, RSC Adv., 2014, 4, 43560 RSC; (g) Y. Ohsedo, M. Oono, K. Saruhashi, H. Watanabe and N. Miyamoto, RSC Adv., 2014, 4, 44837 RSC; (h) Y. Ohsedo, M. Oono, K. Saruhashi and H. Watanabe, RSC Adv., 2014, 4, 48554 RSC; (i) Y. Ohsedo, M. Taniguchi, M. Oono, K. Saruhashi and H. Watanabe, New J. Chem., 2015, 39, 6482 RSC.
- (a) E. A. Wilder, C. K. Hall, S. A. Khan and R. J. Spontak, Langmuir, 2003, 19, 6004 CrossRef CAS; (b) J.-Y. Wang, Z.-H. Wang, J. Gao, L. Wang, Z.-Y. Yang, D.-L. Kong and Z.-M. Yang, J. Mater. Chem., 2009, 19, 7892 RSC; (c) J.-G. Wang, H.-M. Wang, Z.-J. Song, D.-L. Kong, X.-M. Chen and Z.-M. Yang, Colloids Surf., B, 2010, 80, 155 CrossRef CAS PubMed; (d) L. Chen, S. Revel, K. Morris, D. G. Spiller, L. C. Serpell and D. J. Adams, Chem. Commun., 2010, 46, 6738 RSC; (e) W.-C. Lai, J. Phys. Chem. B, 2011, 115, 11029 CrossRef CAS PubMed; (f) W.-C. Lai, S.-J. Tseng and Y.-S. Chao, Langmuir, 2011, 27, 12630 CrossRef CAS PubMed; (g) Y. Zhang, N. Li, J. Delgado, Y. Gao, Y. Kuang, S. Fraden, I. R. Epstein and B. Xu, Langmuir, 2012, 28, 3063 CrossRef CAS PubMed; (h) P. Li, X.-Q. Dou, C.-L. Feng and D. Zhang, Soft Matter, 2013, 9, 3750 RSC; (i) D. J. Cornwell, B. O. Okesola and D. K. Smith, Angew. Chem., Int. Ed., 2014, 53, 12461 CAS.
- M. Shibayama, Polym. J., 2011, 43, 18 CrossRef CAS PubMed.
- (a) Y. Okumura and K. Ito, Adv. Mater., 2001, 13, 485 CrossRef CAS; (b) K. Haraguchi and T. Takehisa, Adv. Mater., 2002, 14, 1120 CrossRef CAS; (c) J. P. Gong, Y. Katsuyama, T. Kurokawa and Y. Osada, Adv. Mater., 2003, 15, 1155 CrossRef CAS PubMed; (d) T. Sakai, T. Matsunaga, Y. Yamamoto, C. Ito, R. Yoshida, S. Suzuki, N. Sasaki, M. Shibayama and U.-I. Chung, Macromolecules, 2008, 41, 5379 CrossRef CAS; (e) A. B. Imran, K. Esaki, H. Gotoh, T. Seki, K. Ito, Y. Sakai and Y. Takeoka, Nat. Commun., 2014, 5, 5124 CrossRef PubMed.
- (a) J.-H. Fuhrhop, P. Schnieder, J. Rosenberg and E. Boekema, J. Am. Chem. Soc., 1987, 109, 3387 CrossRef CAS; (b) J.-H. Fuhrhop and C. Boettcher, J. Am. Chem. Soc., 1990, 112, 1768 CrossRef CAS; (c) J.-H. Fuhrhop, S. Svenson, C. Boettcher, E. Rössler and H.-M. Vieth, J. Am. Chem. Soc., 1990, 112, 4307 CrossRef CAS.
- Mixing alkylglucamine and surfactant created stable molecular hydrogel: (a) J.-H. Fuhrhop, P. Schnieder, E. Boekema and W. Helfrich, J. Am. Chem. Soc., 1988, 110, 2861 CrossRef CAS; (b) L. E. Buerkle, R. Galleguillos and S. J. Rowan, Soft Matter, 2011, 7, 6984 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16823f |
| This journal is © The Royal Society of Chemistry 2015 |

