Dissociation of O2 and its reactivity on O/S doped graphene†
Meiling Houa,
Wanglai Cen*bc,
Fang Nand,
Jianjun Liac,
Yinghao Chu*ac and
Huaqiang Yinac
aCollege of Architecture and Environment, Sichuan University, Chengdu, 610065, P. R. China. E-mail: chuyinghao@scu.edu.cn; Fax: +86 28 85401682; Tel: +86 28 85401682
bInstitute of New Energy and Low Carbon Technology, Sichuan University, Chengdu, 610065, P. R. China. E-mail: cenwanglai@163.com
cNational Engineering Research Center for Flue Gas Desulfurization, Chengdu, 610065, P. R. China
dSichuan Electric Design and Consulting Company, Chengdu, 610094, P. R. China
First published on 28th December 2015
Abstract
It is routinely believed that the oxidation of SO2 to SO3 dominates the removal rate of SO2 on carbon-based catalysts. Recently, both experiment and theoretical calculations evidence that SO2 is readily oxidized by epoxy groups on graphene oxides at room temperature. Based on this fact, we hypothesize in this study that the real rate-determining step for SO2 catalytic oxidation under O2 atmosphere could be the dissociation of molecular O2, which further forms oxygen functional groups on the graphene surface. Density functional theory corrected with dispersion was employed to investigate the dissociation of O2 on O or S doped graphene and then its reactivity for SO2 oxidation. The results showed that O/S doping greatly promotes the dissociation, which leads to the formation of epoxy and/or carbonyl groups on the graphene surface. However, a high oxidation barrier for the oxidation of SO2 by the carbonyl group was found, which implies that the carbonyl group is of low reactivity. Therefore, dopant screening or the design of doped structures should be carefully considered to avoid the formation of carbonyl during O2 dissociation.
1 Introduction
Sulfur dioxide (SO2) is one of the notorious pollutants with toxicity, which is released by the burning of fossil fuels, such as in automobile engines and power plants, and contributes greatly to acid deposition. Great efforts have been made to develop techniques for its treatment. Among them, carbon materials, typically activated carbon (AC) and activated carbon fibres (ACF), have long been known to be general media for the catalytic removal of SO2 at low temperature (20–150 °C).1–4 However, their mechanism at the atomic level remains unclear, which hinders the development of more effective carbon-based catalysts for desulfurization.It is conventionally thought that the rate-determining step for the catalytic removal of SO2 by carbon is the oxidation of SO2 to SO3.5 However, it was found that the oxidation could be catalyzed by graphene oxides (GO) at room temperature.6 In addition, by density functional theory calculations, Zhang and coworkers7,8 found that SO2 can be oxidized by the epoxy groups of GO with a rather low energy barrier. Both experimental and theoretical results indicate that epoxy groups should be the active center for the oxidation of SO2, which might not be the rate-determining step. Alternatively, we hypothesize that the real rate-determining step in the oxidation of SO2 might be the formation of epoxy groups from the dissociation of sluggish O2.
Graphene (GP) can be used as a model catalyst to provide insights for the catalytic oxidation of SO2 on carbon materials.6,9–11 Doping with foreign atoms has been confirmed to be an effective functionalization method.12–16 For example, N-doped graphene was found to be active for the oxygen reduction reaction (ORR).17–19 The ORR is a four-electron pathway, which is believed to depend on the spin density and atomic charge redistribution on the neighboring carbon atoms close to the doping sites.18,20 Besides, Dai21 et al. proposed that the high activity of N-doped GP may be attributed to the larger electronegativity of N, which creates a positive charge on the adjacent C atoms. These results indicate that doping with other electronegative heteroatoms and the charge redistribution caused by them may favor the adsorption and reduction of O2 as well. However, doping with some low electronegative elements, such as boron,15,22 sulfur,23,24 or their mixture,25,26 have also significantly promoted the catalytic activity of GP. The charged sites created by breaking the electroneutrality of GP might be the key factor, regardless of the electronegativity of doping atoms.22
In this study, S and O atoms were separately introduced into GP to investigate the probable dependence of O2 molecule adsorption and dissociation on the electronegativity of the doping atoms, by using density functional theory (DFT) calculations. The electronegativity of the S atom is 2.58, which is close to that of the C atom, while the electronegativity of O is 3.44, which is much stronger than that of the C atom. Both experimental27 and theoretical results27,28 indicate that the substitution of internal atoms in graphene by heteroatoms is the key role for the promoted catalytic activity of graphene materials. Therefore, only the substitution of internal C atom(s) by O/S atoms will be considered. To determine the activity of oxygenic functional groups on doped-graphene, the adsorption and oxidation of SO2 on the doped materials are calculated.
2 Models and methods
2.1 Computational models
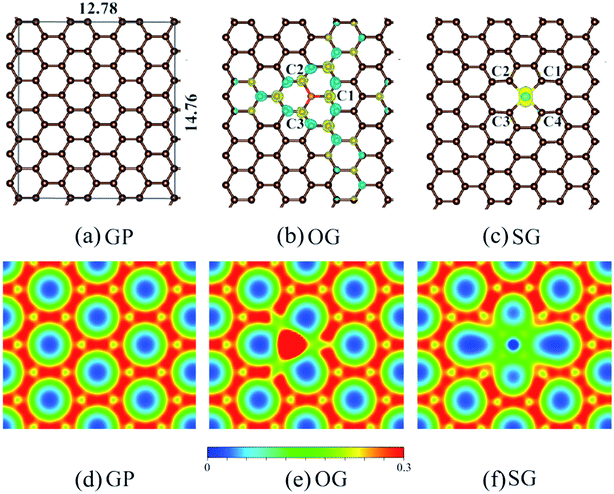
A 3 × 6 graphene unit cell (with 72 carbon atoms, GP) was employed as the model substrate, as depicted in Fig. 1a. It was large enough for the reaction, SO2 + ½O2 → SO3, according to our previous studies.7,8 A vacuum region of 20 Å was added perpendicular to the graphene plane to minimize the interaction between different layers.29 A single O atom took the place of one C atom (Fig. 1b), which is denoted as OG. A single S atom took the place of two neighboring C atoms (Fig. 1c), which is denoted as SG. The relaxed bond lengths of C–C in GP are 1.42 Å, which are consistent with the experimental data. The relaxed C–O bonds and C–S bonds are 1.48 and 1.86 Å, respectively. Although it has been confirmed that S can be doped in the plane of GP, the local structures remain unclear.23 We tested two doping models: a single S atom replacing one and two internal C atom(s). It was found that the latter is more feasible (Fig. S1–S3†). Therefore, only the two C atoms replaced model was used, and is denoted as SG by default.
× 6 graphene unit cell (with 72 carbon atoms, GP) was employed as the model substrate, as depicted in Fig. 1a. It was large enough for the reaction, SO2 + ½O2 → SO3, according to our previous studies.7,8 A vacuum region of 20 Å was added perpendicular to the graphene plane to minimize the interaction between different layers.29 A single O atom took the place of one C atom (Fig. 1b), which is denoted as OG. A single S atom took the place of two neighboring C atoms (Fig. 1c), which is denoted as SG. The relaxed bond lengths of C–C in GP are 1.42 Å, which are consistent with the experimental data. The relaxed C–O bonds and C–S bonds are 1.48 and 1.86 Å, respectively. Although it has been confirmed that S can be doped in the plane of GP, the local structures remain unclear.23 We tested two doping models: a single S atom replacing one and two internal C atom(s). It was found that the latter is more feasible (Fig. S1–S3†). Therefore, only the two C atoms replaced model was used, and is denoted as SG by default.
2.2 Computational methods
All density functional theory (DFT) calculations were carried out with the code VASP5.2 (ref. 30 and 31) using the generalized gradient approximation with a Perdew–Burke–Ernzerhof (PBE) exchange and correlation functional.32 A plane-wave basis set with the cut-off energy of 400 eV was employed within the framework of the projector augmented-wave (PAW) method.33 The Brillouin zone was sampled with 3 × 3 × 1 k-points sampled with Monkhorst–Pack method. Gaussian smearing was used, with a smearing width of 0.2 eV. The D2 method of Grimme34 was used to describe the van der Waals (vdW) correction with default parameters. All the atoms, except those on the boundary, were relaxed and converged to 0.02 eV Å−1. The carbon atoms on the boundary of the GP substrate were frozen in all directions. All the abovementioned parameters were sufficient to ensure that the total energy converged to within 1 meV per atom. The calculated bond lengths of C–C in graphene and O–O in an isolated O2 molecule are 1.42 and 1.26 Å, respectively, which are consistent with published values.35,36 The nudged elastic band (NEB) method37,38 was used to search the minimum reaction pathway (MEP) of O2 reduction by heteroatom-doped graphene from the initial state (IS) to its final state (FS) with 8–12 replicas interpolated. The transition state (TS) was localized with the climbing image method and verified with a single imaginary frequency.The adsorption energy, ΔEads, is defined as follows:
| ΔEads = Etot − (Emol + Esheet) |
3 Results
3.1 S and O doped graphene
We first focus on the electronic structures of pristine and doped-graphene. It is believed that spin density and positive charge can be introduced by electronegative atoms doping, which are of importance for catalytic activity promotion. Spin density determines the positional selectivity of radical adsorption, while charge density determines the adsorption energy.20 Fig. 1b shows that spin density was induced by O doping in an up and down pattern alternately among 1–4 C atoms from the dopant, while the spin was localized at the S atom for SG, as demonstrated in Fig. 1c. The charge density in Fig. 1e illustrates that electrons were transferred from C1–C3 atoms to the dopant O atom, which results in positively charged C atoms for species adsorption. The redistribution of charge densities of C atoms in SG is almost unperceivable. Consequently, the order for the catalytic activity of the three different samples might be GP < SG < OG, and the C atoms directly connected to dopants should be regarded as active sites. Density of states (DOS) analysis showed that the main electron structures of S- and O-doped GP remained as that of the pristine GP (Fig. S4†).3.2 Adsorption and dissociation of O2 molecule
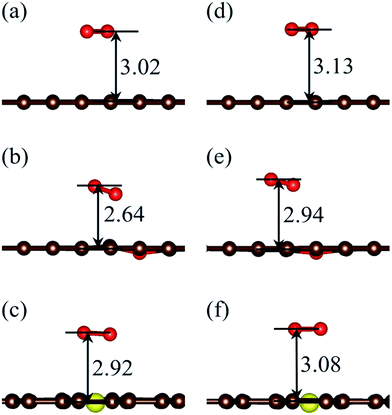 | ||
| Fig. 2 Relaxed adsorption configurations of O2 on GP, OG and SG surfaces. Spin polarization was ignored in (a)–(c), while it was included for (d)–(f). All the distances are given in Å. | ||
As tabulated in Table 1, the adsorption energies obtained with spin polarization are lower than those with non-spin polarization correspondingly, and the adsorption energies increase in the order GP < SG < OG, both of which are consistent with the geometric trends mentioned above. The adsorption energy of O2 on GP in Fig. 2d is 0.14 eV and this is also in good agreement with the previous calculated value of 0.11 eV,39 while it is slightly larger than the experimentally tested value of 0.1 eV.40
Based on the geometric and energetic results, spin polarization possesses few effects and can be ignored for qualitative conclusions.
 | ||
| Fig. 3 Minimum energy pathway (MEP) for the dissociation of oxygen on pristine graphene (GP). All the lengths are given in Å. | ||
The MEP for the oxygen dissociation on doped-GP is demonstrated as Fig. 4 for OG and Fig. 5 for SG. On the approach of O2 to OG (from the IS to MS), the bond length of O2 was elongated from 1.26 to 1.38 Å, where O2 was converted to O2− (superoxide ion). The barrier of this process was about 0.01 eV according to our testing calculations, with a net energy release of 2.04 eV. The C atom connected to the adsorbed O2 was drawn out of the OG surface, which was one of the nearest C atoms originally connected to the doped O atom.
 | ||
| Fig. 4 Minimum energy pathway (MEP) for the dissociation of oxygen on O-doped graphene (OG). All lengths are given in Å. | ||
 | ||
| Fig. 5 Minimum energy pathway (MEP) for the dissociation of oxygen on S-doped graphene (SG). All the lengths are given in Å. | ||
From the MS to FS in Fig. 4, the reaction energy barrier is as low as 0.05 eV, with a net energy release of 1.07 eV. During this process, O2− was pulled closer to the substrate. The bond length of the O–O bond was further elongated from 1.38 Å to 1.41 Å in the TS, and then to 2.68 Å in the FS, which means that the O–O bond in oxygen was thoroughly broken. The determining step appears to be the breaking of the O–O bond of the adsorbed superoxide ion. After the dissociation, one O atom of the O2 molecule was connected with the two C atoms on the OG surface to form an epoxy group, and the other O atom with one C atom to form a carbonyl group. The two bonds of the epoxy are about 1.48 and 1.42 Å, which are in accordance with the calculation results in ref. 8.
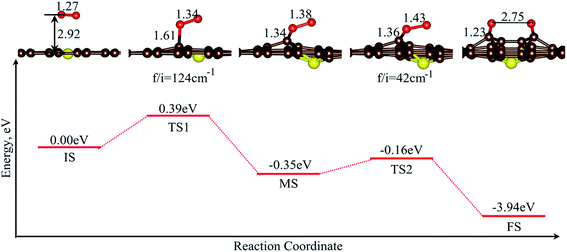
The MEP of O2 dissociation on SG is shown in Fig. 5. Moreover, there are two energy barriers for the dissociation of O2, and obviously the first energy barrier is the main one, with a value of 0.39 eV. Similar to the situation on GP, the determining step was the activation of the adsorbed O2 molecule to form the superoxide ion, with the O–O bond elongated from 1.26 to 1.34 Å. Then, the O–O bond was further elongated, with a barrier of 0.19 eV to form two carbonyl groups (FS), with the O–C bond length of 1.23 Å.
3.3 SO2 oxidation by dissociated O2
It is known that heteroatom doping can promote the dissociation of oxygen on graphene. However, the reactivity of the formed oxygen groups is unknown. Thus, we tested the oxidation of SO2 on two doped-GPs. For conciseness, we denoted the dissociated adsorption configuration of O2 on OG (SG) as 2O_OG (2O_SG). When one oxygen group was consumed through the oxidation reaction, the left species was denoted as 1O_OG (1O_SG). Selected adsorption and reaction energies for the SO2 oxidation are listed in Table 2. Combined with energetic data analysis for the dissociation of O2, it is interesting to find that the two catalytic loops are thermodynamically favorable. However, the rate-determining step for the catalytic oxidation of SO2 by OG is SO2/1O_OG → SO3/OG, with a barrier of 2.46 eV, and that by SG for SO2/2O_SG → SO3/1O_SG is 2.57 eV. Both pathways are not kinetically promising as expected because their rate-determining barriers are unsatisfactory.| Adsorption | ΔEads, eV | Reaction | Eb, eV | Er, eV |
|---|---|---|---|---|
| SO2/2O_OG | −0.35 | SO2/2O_OG → SO3/1O_OG | 0.79 | −1.79 |
| SO2/1O_OG | −0.46 | SO2/1O_OG → SO3/OG | 2.46 | 1.48 |
| SO2/2O_SG | −0.27 | SO2/2O_SG → SO3/1O_SG | 2.57 | 0.62 |
| SO2/1O_SG | −0.37 | SO2/1O_SG → SO3/SG | 1.41 | −0.03 |
According to previous studies,6,8 the epoxy group should be responsible for the high activity of graphene oxides for the oxidation of SO2. When O2 was dissociated on OG, an epoxy group and a carbonyl group were formed in the configuration 2O_OG, while for 2O_SG, there were two carbonyl groups formed. The carbonyl group is supposed to be sluggish for oxidation. The MEP for the oxidation of SO2 by 2O_OG is shown in Fig. 6 to provide some evidence for further understanding the oxidation mechanism.
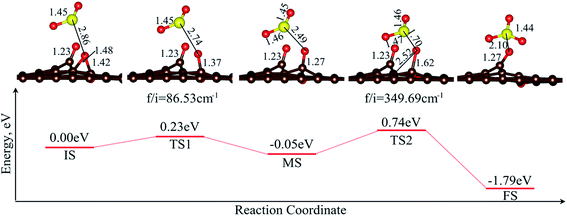 | ||
| Fig. 6 Minimum energy pathway (MEP) for the oxidation of SO2 by 2O_OG. All the lengths are given in Å. | ||
During the first section from the IS to MS, the epoxy group was transferred to the carbonyl group as well, with a small barrier of 0.23 eV. The oxidation barrier from the MS to FS was 0.79 eV, with a net energy release of 1.79 eV. Such a high barrier confirms that the carbonyl group is inertial for oxidation. This is quite different for the oxidation of SO2 by the epoxy group located on pristine GP,8 where the barrier is as low as ca. 0.2 eV. The main difference should be the extension of the O–C bond during oxidation. For the epoxy group, the O–C bond was extended from 1.48 to 1.81 Å, with an extension of 0.33 Å, while for the carbonyl group, the O–C bond was extended from 1.27 to 1.62 Å, with an extension of 0.35 Å. The shorter initial O–C bond length and the longer extension result in a much higher oxidation barrier. Accordingly, it is rationally expected that the barriers for the oxidation processes SO2/1O_OG → SO3/OG, SO2/2O_SG → SO3/1O_SG and SO2/1O_SG → SO3/SG are also much higher, as carbonyl groups were involved.
4 Discussion
It has been found that the dissociation of O2 on graphene can be efficiently promoted by heteroatom doping; however, the oxidation activities of oxygen species derived from O2 dissociation are quite different and still unsatisfactory. Herein, attention is paid to the electronic structures of different oxygen species to obtain a fundamental understanding.As shown in Fig. 7, compared with the situation of the epoxy group on GP (Fig. 7a), the valence band of the epoxy group on OG (Fig. 7b) is shifted to a lower level. Besides the shift of the primary highest occupied molecular orbital (HOMO, marked as A in the plot), a new peak (marked as B) appears, as presented in Fig. 7b. This implies that the epoxy group of OG is more inertial than that of GP. Fig. 7c shows a low level peak (marked as C), which is present below −20 eV. This confirms that the carbonyl group is considerably sluggish for oxidation, because the cost of energy is prohibitive to activate it.
 | ||
| Fig. 7 Density of states (DOS) analysis for oxygen functional groups on different surfaces. (a) Epoxy group on GP surfaces, (b) epoxy group on OG surfaces, and (c) carbonyl group on SG surface. | ||
Based on electronic structure analysis, it was found that the formation of the highly active epoxy group from O2 dissociation should be the key point for the design and synthesis of graphene-based redox catalysts. In the present study, both O and S doping resulted in the formation of a carbonyl group, which is inertial in the catalytic oxidation process. Other dopants or doping patterns might be more promising.
5 Conclusion
Density functional theory corrected with dispersion was used to investigate the potential promotion effects of O and S doping in graphene on oxygen dissociation and catalytic oxidation of SO2. It was found that O/S doping tremendously promotes the dissociation of sluggish molecular oxygen, which leads to the formation of epoxy and/or carbonyl groups on the doped graphene surfaces. Due to the formation of carbonyl group, the catalytic loop for the oxidation of SO2 is terminated because of its high oxidation barrier. These results indicate that more attention should be paid on dopant screening or the design of doped structures to avoid the formation of carbonyl in O2 dissociation. Further exploration is needed to screen other dopants or doping patterns.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (51508356, 51378325) and Young Talent Program for Science and Technology Innovation of Sichuan Province in China (2015MZGC001). We also acknowledge the National Supercomputer Center in Shenzhen of China for computational service support.References
- X. Liu, J. Guo, Y. Chu, D. Luo, H. Yin, M. Sun and R. Yavuz, Desulfurization performance of iron supported on activated carbon, Fuel, 2014, 123, 93–100 CrossRef CAS
.
- Y. Qu, J. Guo, Y. Chu, M. Sun and H. Yin, The influence of Mn species on the SO2 removal of Mn-based activated carbon catalysts, Appl. Surf. Sci., 2013, 282, 425–431 CrossRef CAS
.
- D. López, R. Buitrago, A. Sepúlveda-Escribano, F. Rodríguez-Reinoso and F. Mondragón, Low temperature catalytic adsorption of SO2 on activated carbon, J. Phys. Chem. C, 2008, 112, 15335–15340 Search PubMed
.
- N. Karatepe, I. Orbak, R. Yavuz and A. Ozyuguran, Sulfur dioxide adsorption by activated carbons having different textural and chemical properties, Fuel, 2008, 87, 3207–3215 CrossRef CAS
.
- A. A. Lizzio and J. A. DeBarr, Mechanism of SO2 removal by carbon, Energy Fuels, 1997, 11, 284–291 CrossRef CAS
.
- Y. Long, C. Zhang, X. Wang, J. Gao, W. Wang and Y. Liu, Oxidation of SO2 to SO3 catalyzed by graphene oxide foams, J. Mater. Chem., 2011, 21, 13934 RSC
.
- H. Zhang, W. Cen, J. Liu, J. Guo, H. Yin and P. Ning, Adsorption and oxidation of SO2 by graphene oxides: A van der Waals density functional theory study, Appl. Surf. Sci., 2015, 324, 61–67 CrossRef CAS
.
- W. Cen, M. Hou, J. Liu, S. Yuan, Y. Liu and Y. Chu, Oxidation of SO2 and NO by epoxy groups on graphene oxides: the role of the hydroxyl group, RSC Adv., 2015, 5, 22802–22810 RSC
.
- C. Huang, C. Li and G. Shi, Graphene based catalysts, Energy Environ. Sci., 2012, 5, 8848–8868 CAS
.
- S. Tang and Z. Cao, Adsorption of nitrogen oxides on graphene and graphene oxides: Insights from density functional calculations, Phys. Chem. Chem. Phys., 2011, 134, 044710 Search PubMed
.
- M. Hou, W. Cen, H. Zhang, J. Liu, H. Yin and F. Wei, Adsorption and oxidation of NO on graphene oxides: A dispersion corrected density functional theory investigation, Appl. Surf. Sci., 2015, 339, 55–61 CrossRef CAS
.
- Z. Mo, R. Zheng, H. Peng, H. Liang and S. Liao, Nitrogen-doped graphene prepared by a transfer doping approach for the oxygen reduction reaction application, J. Power Sources, 2014, 245, 801–807 CrossRef CAS
.
- J. Bai, Q. Zhu, Z. Lv, H. Dong, J. Yu and L. Dong, Nitrogen-doped graphene as catalysts and catalyst supports for oxygen reduction in both acidic and alkaline solutions, Int. J. Hydrogen Energy, 2013, 38, 1413–1418 CrossRef CAS
.
- Z. Ma, S. Dou, A. Shen, L. Tao, L. Dai and S. Wang, Sulfur-dopend graphene derived from cycled lithium–sulfur batteries as a metal-free electrocatalyst for the oxygen reduction reaction, Angew. Chem., Int. Ed., 2015, 54, 1888–1892 CrossRef CAS PubMed
.
- S. Wang, L. Zhang, Z. Xia, A. Roy, D. Chang, J. B. Baek and L. Dai, BCN Graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction, Angew. Chem., Int. Ed., 2012, 51, 4209–4212 CrossRef CAS PubMed
.
- M. Li, L. Zhang, Q. Xu, J. Niu and Z. Xia, N-doped graphene as catalysts for oxygen reduction and oxygen evolution reactions: Theoretical considerations, J. Catal., 2014, 314, 66–72 CrossRef CAS
.
- C. H. Choi, M. W. Chung, H. C. Kwon, J. H. Chung and S. I. Woo, Nitrogen-doped graphene/carbon nanotube self-assembly for efficient oxygen reduction reaction in acid media, Appl. Catal., B, 2014, 144, 760–766 CrossRef CAS
.
- L. Zhang and Z. Xia, Mechanisms of oxygen reduction reaction on nitrogen-hoped graphene for fuel cells, J. Phys. Chem. C, 2011, 115, 11170–11176 CAS
.
- Q. Liu, H. Zhang, H. Zhong, S. Zhang and S. Chen, N-doped graphene/carbon composite as non-precious metal electrocatalyst for oxygen reduction reaction, Electrochim. Acta, 2012, 81, 313–320 CrossRef CAS
.
- L. Zhang, J. Niu, L. Dai and Z. Xia, Effect of microstructure of nitrogen-doped graphene on oxygen reduction activity in fuel cells, Langmuir, 2012, 28, 7542–7550 CrossRef CAS PubMed
.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen, Science, 2009, 323, 760–763 CrossRef CAS PubMed
.
- L. Yang, S. Jiang, Y. Yu, L. Zhu, S. Chen, X. Wang, Q. Wu, J. Ma, Y. Ma and Z. Hu, Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction, Angew. Chem., Int. Ed., 2011, 50, 7132–7135 CrossRef CAS PubMed
.
- Z. Yang, Z. Yao, G. Li, G. Fang, H. Nie, L. Zheng, X. Zhou, X. Chen and S. Huang, Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction, ACS Nano, 2012, 6, 205–211 CrossRef CAS PubMed
.
- I. Y. Jeon, S. Zhang, L. Zhang, H. J. Choi, J. M. Seo, Z. Xia, L. Dai and J. B. Baek, Edge-selectively sulfurized graphene nanoplatelets as efficient metal-free electrocatalysts for oxygen reduction reaction: The electron spin effect, Adv. Mater., 2013, 25, 6138–6145 CrossRef CAS PubMed
.
- J. Liang, Y. Jiao, M. Jaroniec and S. Qiao, Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance, Angew. Chem., Int. Ed., 2012, 51, 11496–11500 CrossRef CAS PubMed
.
- Y. Zheng, Y. Jiao, L. Ge, M. Jaroniec and S. Qiao, Two-step boron and nitrogen doping in graphene for an enhanced synergistic catalysis, Angew. Chem., Int. Ed., 2013, 52, 3110–3116 CrossRef CAS PubMed
.
- Y. Gao, G. Hu, J. Zhong, Z. Shi, Y. Zhu, D. S. Su, J. Wang, X. Bao and D. Ma, Nitrogen-doped sp2-hybridized carbon as a superior catalyst for selective oxidation, Angew. Chem., Int. Ed., 2013, 52, 2109–2113 CrossRef CAS PubMed
.
- D. W. Boukhvalov, Y. W. Son and R. S. Ruoff, Water splitting over graphene-based catalysts: Ab initio calculations, ACS Catal., 2014, 4, 2016–2021 CrossRef CAS
.
- M. Yang, M. Zhou, A. Zhang and C. Zhang, Graphene oxide: An ideal support for gold nanocatalysts, J. Phys. Chem. C, 2012, 116, 22336–22340 CAS
.
- G. Kresse and J. Furthmuller, Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS
.
- G. Kresse and J. Furthmuller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS
.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed
.
- G. Kresse and D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS
.
- S. Grimme, Semiempirical GGA-type density functional constructed with a long-range dispersion correction, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed
.
- D. R. Lide, Handbook of chemistry and physics, CRC Press, 84th edn, 2003 Search PubMed
.
- E. Fuente, J. A. Menendez, M. A. Diez, D. Suarez and M. A. Montes-Moran, Infrared spectroscopy of carbon materials: A quantum chemical study of model compounds, J. Phys. Chem. B, 2003, 107, 6350–6359 CrossRef CAS
.
- G. Henkelman and H. Jonsson, Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points, J. Chem. Phys., 2000, 113, 9978–9985 CrossRef CAS
.
- G. Henkelman, B. P. Uberuaga and H. Jonsson, A climbing image nudged elastic band method for finding saddle points and minimum energy paths, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS
.
- A. Pramanik and H. S. Kang, Density functional theory study of O2 and NO adsorption on heteroatom-doped graphene including the van der Waals interaction, J. Phys. Chem. C, 2011, 115, 10971–10978 CAS
.
- G. Vidali and G. Ihm, Potentials of physical adsorption, Surf. Sci. Rep., 1991, 12, 133–181 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16789b |
| This journal is © The Royal Society of Chemistry 2016 |