Reduced surface defects of organometallic perovskite by thermal annealing for highly efficient perovskite solar cells
Abstract
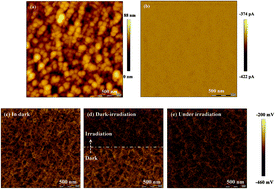
The surface defects of the organometallic perovskite play an important role in the photovoltaic performance of solar cells, which depress the conversion efficiency and cause photocurrent hysteresis. As a key step in fabricating perovskite solar cells, the heating step possibly modifies the surface defects, which in turn leads to the modified device performance. In this context, the surface defects of the organometallic perovskite (CH3NH3PbI3) and their modification by thermal annealing are investigated. It is revealed that the surface defects create electron traps which can be reduced by thermal annealing. Consequently, the perovskite solar cells exhibit improved conversion efficiency from 10.9% to 17.1% with the thermal annealing temperature of the perovskite increasing from 60 °C to 130 °C. The photocurrent hysteresis of the solar cells is also subdued. This work provides further insights into the function of thermal annealing by modifying surface defects, which also favors the exploration of the perovskite solar cells with high-efficiency and eliminated photocurrent hysteresis.

 Please wait while we load your content...
Please wait while we load your content...