In silico binding affinity to cyclooxygenase-II and green synthesis of benzylpyrazolyl coumarin derivatives†
Arijit Saha a,
Soumen Payraa,
Sant Kumar Vermab,
Mou Mandala,
Suresh Tharejab and
Subhash Banerjee*a
a,
Soumen Payraa,
Sant Kumar Vermab,
Mou Mandala,
Suresh Tharejab and
Subhash Banerjee*a
aDepartment of Chemistry, Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur-495009, Chhattisgarh, India. E-mail: ocsb2006@gmail.com
bSchool of Pharmaceutical Sciences, Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur-495009, Chhattisgarh, India
First published on 19th November 2015
Abstract
Here, we have reported for the first time, in silico binding affinity prediction of benzylpyrazolyl coumarin scaffolds to cyclooxygenase-II (COX-II) enzyme and a green synthetic route to access these scaffolds using a neutral ionic liquid, [pmIm]Br as the reusable catalyst and reaction media under metal-free conditions. The integrated scaffolds, benzylpyrazolyl coumarins exhibited stronger binding affinity to COX-II inhibitor than their individual moieties, 3-benzyl coumarins and pyrazolones. Moreover, 4-((4-hydroxy-2-oxo-2H-chromen-3-yl)(4-nitrophenyl)methyl)-5-methyl-2-phenyl-1H-pyrazol-3(2H)-one (3c) has shown even better binding affinity than the marketed anti-inflammatory drug, celecoxib.
The design and development of integrated molecular scaffolds for biological applications have attracted much attention as these moieties exhibit wide and improved bio-activities. In this connection, 3-benzyl substituted coumarins are of much interest as these moieties show a broad range of biological activities and consequently these moieties are found in many natural products namely as warfarin, phenprocumene and coumatetralyl which have been reported to show antibacterial, anti-HIV,1a antiviral,1b anticoagulant,1c antioxidant1d and anticancer activities1e (Fig. 1a). On the other hand, pyrazolones also exhibit a broad spectrum of biological activities and a few of them for example, phenazone, propyphenazone, ampyrone and edarvone are well known as antipyretic and analgesic drugs (Fig. 1b).2 In addition, pyrazolones are also recognized as antimicrobial, antifungal,3a antimycobacterial,3b antibacterial,3c anti-inflammatory,3d,e antitumor,3f gastric secretion stimulatory,3g antidepressant,3h antifilarial activities3i and anti-tubercular activities.3j It is anticipated that the integration of the 3-benzyl coumarin and pyrazolone moieties leading to benzylpyrazolyl coumarin scaffolds (Fig. 1c) could be fascinating and beneficial from the biological point of view. However, interestingly, binding affinity and selectivity of benzylpyrazolyl coumarin derivatives against any biological target has not been investigated.
 | ||
| Fig. 1 Some bioactive individual (a) 3-benzyl substituted coumarins, (b) pyrazolones and (c) model integrated scaffold. | ||
Over past decades, nonsteroidal anti-inflammatory drugs (NSAIDs) are of enormous importance as therapeutics for the treatment of pain and inflammation, predominantly arthritis.4 In this connection, design and development of NSAIDs are of much importance to overcome the general limitations of present drugs such as GI bleeding, ulceration, perforation, and obstruction etc.5
The major pharmacological mechanism of action of NSAIDs is the enzymatic inhibition of cyclooxygenase (COX) mediated production of pro-inflammatory prostaglandins and thromboxanes. COX and lipoxygenase (LOX) are the key enzymes which catalyze the rate-limiting steps in the biosynthesis of prostaglandins and leukotrienes from arachidonic acid.6 The two different forms of the COX enzymes are COX-I (constitutive) and COX-II (inducible), respectively. COX-I is expressed in many organs and is responsible for homeostatic processes such as platelet aggregation, gastric protection, and renal function while COX-II enzyme induced in response to the release of several pro-inflammatory mediators leading to the inflammatory response and pain.7 However, simultaneous inhibition of COX-I results the gastrointestinal toxicities and the mild bleeding diathesis.8 Whereas, COX-II enzyme was found to be anti-inflammatory, responsible for the selective inhibition but non-ulcerogenic in both the carrageenin-induced inflammation and the adjuvant arthritis.9 Therefore, the research in the field of inflammation around the world relies on the search of potent, selective and specific inhibitors of the COX-II.
Computer aided drug design tools have been widely used for the prediction of biological activity, toxicity, binding modes, selectivity and screening of a library of synthetic compounds against a particular biological target. It is evident from the literature that the pyrazolones widely exhibits COX-II inhibitory activity.3d,e Here, we have evaluated their binding affinity against COX-II inhibitor enzyme of integrated benzylpyrazolyl coumarin scaffolds (3a–g, Fig. 2) and compared with those of individual moieties of pyrazolones (1a, b, Fig. 2) and 3-benzyl coumarins (2a–e, Fig. 2).
 | ||
| Fig. 2 Structure of individual pyrazolones (1a, b), 3-benzyl coumarins (2a–e) and their integrated scaffold, benzylpyrazolyl coumarins (3a–g). | ||
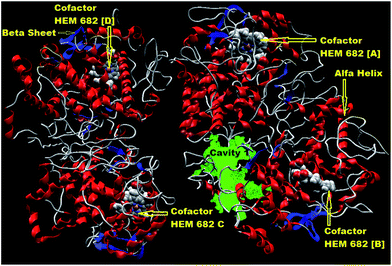
Molecular docking technique is used in the present study for the comparison of binding affinity and selectivity of parent scaffolds and their integrated complex against COX-II. Molegro virtual docker (MVD 2013.6.0.0 evaluation version) was used for the flexible docking study of parent scaffolds and their union complexes with COX-II which is based on molecular docking simulations such as intramolecular interaction energy of the ligand (Eintra), ligand and macromolecular interaction energy i.e. intermolecular interaction energy (Einter) and docking scoring function (Escore). Initially, for performing molecular docking, the 3D structures of COX-II, molecular mechanics (MM2) and Hamiltonian approximation (AM1) optimized (both the optimizers were run till the root-mean-square gradient value reaches a value smaller than 0.001 kcal mol−1 Å−1) parent scaffolds and their union complexes were imported into the workspace. During import process, intense care should be taken that all the crystallographic water molecules were removed from COX-II. Further, COX-II and all the ligands were subjected to molecules preparation. The secondary structure of COX-II with co-factors is presented in Fig. 3. Five distinct cavities (see Fig. S1 in ESI-1†) with different surface area and volume have been mapped within COX-II (PDB Id.: 4COX) using detect cavity module in MVD software.10
During this computational process, the maximum numbers of cavities were fixed to 5, grid resolution 0.80 Å and probe size 1.2 Å; while the other parameters were kept as default. The volume and surface area of these cavities are depicted in Table 1.
| Cavity no. | Volume (Å3) | Surface area (Å2) |
|---|---|---|
| 1 | 1561.090 | 3776.000 |
| 2 | 1426.940 | 3566.080 |
| 3 | 1370.620 | 3257.600 |
| 4 | 698.880 | 1539.840 |
| 5 | 242.688 | 807.680 |
The cavity 1 (Fig. 5) has highest volume (1561.090 Å3) along with largest surface area (3776.000 Å2). The key residues of cavity 1 included Ala 151, Arg 44, Arg 469, Asn 39, Asn 43, Asp 125, Cys 36, Cys 47, Gln 42, Gln 46, Glu 46, Glu 465, Gly 45, Leu 152, Lys 468, Lys 546, Pro 40, Pro 153, Ser 38, Ser 471, Tyr 130, Tyr 466 (Fig. 4). For performing docking the option docking wizard in docking window was selected and the docking algorithm was set to MolDock Simplex Evolution (MolDock SE) with population size 50. RMSD thresholds were set 1.00 Å for cluster similar poses as well as for ignore similar poses (for multiple runs only) both. Number of independent runs were selected ten and each of these runs was returning to a single final solution i.e. pose. Only negative lowest-energy representative cluster was returned from each of them after completion of docking and the similar poses were removed keeping the best scoring one. The clusters were ranked through compare the conformation of the lowest binding energy in each cluster. The first minimum binding free energy pose was selected for the analysis of the docking results. Other parameters such as binding radius, grid resolution and maximum iterations parameters were set to 15 Å, 0.3 Å and 1500 respectively.11
Further, MolDock score, re-rank score and H-bond score, obtained after docking simulation are the markers of the affinity of the parent scaffolds and their integration complexes with COX-II (Table 2). These scores permit us to recognize the significant interactions take place between different conformations of each ligand and the amino acid residues present in active site of COX-II in order to get best minimum energy conformation of each ligand.12
| Ligand | MolDock score | H-bond score | Re-rank score |
|---|---|---|---|
| 1a | −58.282 | −3.182 | −47.830 |
| 2a | −105.935 | 0.000 | −92.001 |
| 3a | −134.877 | −6.372 | −104.615 |
| 1b | −92.549 | −2.294 | −80.324 |
| 2a | −105.935 | 0.000 | −92.001 |
| 3b | −145.568 | −4.964 | −118.887 |
| 1b | −92.549 | −2.294 | −80.324 |
| 2b | −124.023 | −5.744 | −106.286 |
| 3c | −162.027 | −3.399 | −105.214 |
| 1b | −92.549 | −2.294 | −80.324 |
| 2c | −113.784 | −2.076 | −98.150 |
| 3d | −154.438 | −1.524 | −113.761 |
| 1b | −92.549 | −2.294 | −80.324 |
| 2d | −118.953 | −1.560 | −101.978 |
| 3e | −150.385 | −6.167 | −124.305 |
| 1b | −92.549 | −2.294 | −80.324 |
| 2e | −118.182 | −3.627 | −97.152 |
| 3f | −154.521 | −0.238 | −124.316 |
| 1a | −58.282 | −3.182 | −47.830 |
| 2e | −118.182 | −3.627 | −97.152 |
| 3g | −152.536 | −5.173 | −114.596 |
| Celecoxib | −156.232 | −2.680 | −126.570 |
It is sighted from the Table 2 that the MolDock scores and re-rank scores of all the integration complexes (3a–g) were greater than that of their parent scaffolds. The compound, 3c demonstrated highest MolDock score (−162.027) with comparable re-rank score (−105.214) which are even greater than celecoxib, a marketed selective COX-II inhibitor and signifies its better affinity and selectivity towards COX-II (Table 2). The hydrogen bond and steric interactions of 3c with COX-II is depicted in Fig. 5 and 6 respectively.
 | ||
| Fig. 5 Hydrogen bonds labeled in dash bonds as 1 and 2 (A) and steric (B) interactions of 3c with COX-II. | ||
The integrated molecular scaffolds possess diverse range of functionalities (e.g. nitro, methoxy, keto, hydroxy, phenyl etc.) for the desired H-bonding and electrostatic interactions with the amino acid moieties of COX-II enzyme, which results significant enhancement of binding affinity and selectivity of integrated benzylpyrazolyl coumarin derivatives to COX-II inhibitor compared to individual moieties.
Thus, the computer aided modeling data successfully demonstrated that the integrated scaffolds (3a–g) selectively and specifically binds with the amino acid residues present in the cavity of COX-II inhibitor to a greater extent compared to the individual moieties (1a, b and 2a–e). Motivated by these results, we have tried to establish a green synthetic route to access these scaffolds (3a–g) under neutral and metal-free reaction conditions. The literature reports indicate that only three methods are available for the synthesis of benzyl pyrazolyl coumarin scaffolds13a–c (Fig. 1c). These methods have utilized either glacial acetic acid,13a or lanthanide13b/alkaline earth13c metal as catalyst. Thus, development of a metal-free green synthetic protocol for the synthesis of scaffold, 3 is highly desirable.
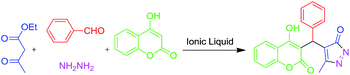
Over decades, ionic liquids (ILs) were reported to act as green alternative reaction media14 and efficient catalysts15 in organic synthesis. Here, we have observed remarkable catalytic activity of a neutral IL, pentyl methyl imidazolium bromide, [pmIm]Br for the one-pot four component synthesis of benzylpyrazolyl coumarin derivatives at room temperature (Scheme 1).
Here, [pmIm]Br acts as both catalyst and reaction medium and no other metal catalysts or organic solvents were used for this synthesis.
Initially, we have chosen synthesis of 4-((4-hydroxy-2-oxo-2H-chromen-3-yl)(phenyl)methyl)-5-methyl-3H-pyrazol-3-one via a four component reaction of benzaldehyde, ethyl acetoacetate, hydrazine hydrate and 4-hydroxy coumarin as a model reaction. When a stoichiometric amount of four components mixed in [pmIm]Br (2 ml) at room temperature for 2 h good yield (85%) of product was obtained (entry 1, Table 3). The same reaction was failed to produce desired product even at elevated temperature (80 °C) without ionic liquid under neat or in water (entry 2, Table 3).
| Entry | Ionic liquid | Time (h) | Yielda (%) |
|---|---|---|---|
| a Isolated yield.b Heating at 70 °C.c 1 ml of [pmIm]Br was used.d Reaction conditions: ethyl acetoacetate (1 mmol), phenyl hydrazine (1 mmol), benzaldehyde (1 mmol), 4-hydroxycoumarin (1 mmol), ionic liquid (2 ml), room temperature, continuous stirring. | |||
| 1 | [pmIm]Br | 2 | 85 |
| 2 | No ionic liquid or H2O/80 °C | 4 | — |
| 3 | [AcMIm]Cl | 2 | 35 |
| 4 | [AcMIm]Clb | 2 | 69 |
| 5 | [pmIm]Brc | 2 | 74 |
Using acidic ionic liquid, [AcmIm]Cl only 35% product was isolated at room temperature (entry 3, Table 3). However, the yield has increased to 69% at 70 °C (entry 4, Table 3). Thus, the reaction using neutral ionic liquid, [pmIm]Br at room temperature was considered as optimized conditions for the synthesis of benzylpyrazolyl coumarin derivatives.
Next, we have explored the scope of this protocol for the generation of a library of benzylpyrazolyl coumarin derivatives. The results were listed in Table 4. A variety of aryl aldehydes containing different substituent (e.g. –NO2, –Cl, –OMe, –Me etc.) participated in this multicomponent reaction to provide the excellent yields (85–90%) of products within practical time period (2 h) and electronic effect of the substituent present in aromatic aldehyde was not observed.
| Entry | (3a–g) | Time (h) | Yielda (%) | Melting point (°C) | |
|---|---|---|---|---|---|
| Observed | Reportedref | ||||
| a Isolated yield.b Reaction condition: ethyl acetoacetate (1 mmol), hydrazine hydrate/phenyl hydrazine (1 mmol), 4-hydroxycoumarin (1 mmol), aryl aldehyde (1 mmol), [pmIm]Br (2 ml), room temperature with continuous stirring. | |||||
| 1 |  |
2.0 | 85 | 229–231 | 231–233 (ref. 13a) |
| 2 |  |
2.5 | 85 | 232–234 | 232–234 (ref. 13a) |
| 3 |  |
2.0 | 87 | 248–249 | 248–250 (ref. 13a) |
| 4 |  |
2.0 | 88 | 228–230 | 227–229 (ref. 13a) |
| 5 |  |
2.5 | 86 | 223–225 | 222–224 (ref. 13a) |
| 6 |  |
2.5 | 85 | 204–206 | 205–207 (ref. 13a) |
| 7 |  |
2.0 | 90 | 200–201 | 202–204 (ref. 13a) |
However, slight lower yields of the products were observed in reactions when phenyl hydrazine is used in place of hydrazine. This could be due to the delocalization of lone pair of one nitrogen atom to the phenyl ring. After the reactions the compounds were separated from ionic liquids and recrystalized from hot ethanol and characterized primarily by checking their melting points and compared these values with the reported values. Further, the structure and purity of compounds were re-confirmed by 1H NMR and 13C NMR studies (ESI-6†) as well as high performance liquid chromatography (HPLC) study (see ESI-7†).
In order to compare the results of synthesis of benzylpyrazolyl coumarin using [pmIm]Br with those of reported protocols, a comparative study of catalytic performance is presented in Table 5. This study clearly indicates that the present protocol better alternative to those of existing as present protocol avoided metal catalysts.
The synthesis of benzylpyrazolyl coumarins proceeds through the catalytic cycle involving simultaneous synthesis of two intermediates, pyrazolone(I) and 3-arylidenechroman-2,4-dione(II) from general condensation and Knoevenagel condensation reaction respectively. Subsequently, the two intermediates react to each other via Michael addition followed by cyclization and tautomerization to afford the benzylpyrazolyl coumarin derivatives. A plausible mechanistic pathway has been presented in ESI-4.†
Conclusions
In conclusion, we have demonstrated enhanced target specific molecular binding affinities of integrated molecular scaffolds, benzylpyrazolyl coumarins with COX-II inhibitor. Moreover, it is noteworthy that the 4-((4-hydroxy-2-oxo-2H-chromen-3-yl)(4-nitrophenyl)methyl)-5-methyl-2-phenyl-1H-pyrazol-3(2H)-one (3c) moiety has exhibited even better affinity in terms of H-bonds score and re-rank score than marketed anti-inflammatory drug, celecoxib. Additionally, here we have developed a green and synthetic methodology to access benzylpyrazolyl coumarin moieties via a facile one-pot multicomponent reaction using a neutral ionic liquid at room temperature. Here, ionic liquids act as both catalyst and reaction medium and of course performed the reactions under neutral conditions. Finally, these scaffolds may further be used for the development of potential and COX-II specific anti-inflammatory agents.Acknowledgements
We are pleased to acknowledge the funding agencies Department of Science and Technology, New Delhi, Govt. of India (NO.SB/FT/CS-023/2012). AS and SP thank to Guru Ghasidas Vishwavidyalaya and SKV thanks ICMR for their fellowship. We are also thankful to Dr Rene Thomsen for providing permission to use MVD software, Prof. B. C. Ranu and Dr K. V. S. Ranganath and their groups for helping in NMR and HPLC studied.Notes and references
- (a) S. Hesse and G. Kirsch, Tetrahedron Lett., 2002, 43, 1213 CrossRef CAS; (b) B. H. Lee, M. F. Clothier, F. E. Dutton, G. A. Conder and S. S. Johnson, Bioorg. Med. Chem. Lett., 1998, 8, 3317 CrossRef CAS PubMed; (c) J.-C. Jung, Y.-J. Jung and O.-S. Park, Synth. Commun., 2001, 31, 1195 CrossRef CAS; (d) G. Melagraki, A. Afantitis, O. Igglessi-Markopoulou, A. Detsi, M. Koufaki, C. Kontogiorgis and D. J. Hadjipavlou-Litina, Eur. J. Med. Chem., 2009, 44, 3020 CrossRef CAS PubMed; (e) J.-C. Jung, J.-H. Lee, S. Oh, J.-G. Lee and O.-S. Park, Bioorg. Med. Chem. Lett., 2004, 14, 5527 CrossRef CAS PubMed.
- T. W. Wu, L. H. Zeng, J. Wu and K. P. Fung, Life Sci., 2002, 71, 2249 CrossRef CAS PubMed.
- (a) M. A. Al-Haiza, S. A. El-Assiery and G. H. Sayed, Acta Pharm., 2001, 51, 251 CAS; (b) D. Castagnolo, F. Manetti, M. Radi, B. Bechi, M. Pagano, A. de Logu, R. Meleddu, M. Saddi and M. Botta, Bioorg. Med. Chem., 2009, 17, 5716 CrossRef CAS PubMed; (c) F. Moreau, N. Desroy, J. M. Genevard, V. Vongsouthi, V. Gerusz, G. LemFralliec, C. Oliveira, S. Floquet, A. Denis, S. Escaich, K. Wolf, M. Busemann and A. Aschenbsenner, Bioorg. Med. Chem. Lett., 2008, 18, 4022 CrossRef CAS PubMed; (d) E. A. M. Badawey and I. M. El-Ashmawey, Eur. J. Med. Chem., 1998, 33, 349 CrossRef CAS; (e) M. E. A. Zaki, H. A. Saliman, O. A. Hickal and A. E. Z. Rashad, Z. Naturforsch., C: J. Biosci., 2006, 61, 1 CrossRef CAS; (f) F. A. Pasha, M. Muddassar, M. M. Neaz and S. J. Cho, J. Mol. Graphics Modell., 2009, 28, 54 CrossRef CAS PubMed; (g) C. E. Rosiere and M. I. Grossman, Science, 1951, 113, 651 CAS; (h) D. M. Bailey, P. E. Hansen, A. G. Hlavac, E. R. Baizman, J. Pearl, A. F. Defelice and M. E. Feigenson, J. Med. Chem., 1985, 28, 256 CrossRef CAS PubMed; (i) P. M. S. Chauhan, S. Singh and R. K. Chatterjee, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1993, 32, 858 Search PubMed; (j) P. Gunasekaran, S. Perumal, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2011, 46, 4530 CrossRef CAS PubMed.
- K. Rullah, M. F. F. M. Aluwi, B. M. Yamin, M. S. Baharuddin, N. H. Ismail, H. Y. Teruna, S. N. A. Bukhari, I. Jantan, J. Jalil, K. Husain and L. K. Wai, J. Mol. Biol., 2015, 1081, 51 CAS.
- (a) G. Singh, D. Ramey, D. Morfeld, H. Shi, H. T. Hatoum and J. F. Fries, Arch. Intern. Med., 1996, 156, 1530 CrossRef CAS; (b) F. K. L. Chan, Nat. Clin. Pract. Gastroenterol. Hepatol., 2006, 3, 563 CrossRef CAS PubMed.
- A. Kumar, B. Ahmed, B. Srivastawa and Vaishali, Der Pharma Chemica, 2012, 4, 383 Search PubMed.
- (a) M. Amir, H. Kumar and S. A. Javed, Eur. J. Med. Chem., 2008, 43, 2056 CrossRef CAS PubMed; (b) M. F. E. Shehry, A. A. Abu-Hashem and E. M. E. Telbani, Eur. J. Med. Chem., 2010, 45, 1906 CrossRef PubMed.
- G. A. FitzGerald and C. N. Patrono, N. Engl. J. Med., 2001, 345, 433 CrossRef CAS PubMed.
- (a) J. L. Masferrer, B. S. Zweifel and P. T. Manning, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 3228 CrossRef CAS PubMed; (b) G. D. Anderson, S. D. Hauser, K. L. McGarity, M. E. Bremer, P. C. Isakson and S. A. Gregory, J. Clin. Invest., 1996, 97, 2672 CrossRef CAS PubMed.
- Protein data bank, An information portal to biological macromolecular structures, http://www.rcsb.org/pdb/explore/explore.do?structureId=4COX, accessed on: July 27, 2015.
- J. Sochacka and B. Pawelczak, Acta Poloniae Pharmaceutica–Drug Research, 2012, 69, 161 CAS.
- R. Thomsen and M. H. Christensen, J. Med. Chem., 2006, 49, 3315 CrossRef CAS PubMed.
- (a) P. P. Ghosh, G. Pal, S. Pal and A. R. Das, Green Chem., 2012, 14, 2691 RSC; (b) A. Saha, S. Payra and S. Banerjee, Green Chem., 2015, 17, 2859 RSC; (c) S. Yaragorla, A. Pareek and R. Dada, Tetrahedron Lett., 2015, 56, 4770 CrossRef CAS.
- (a) T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed; (b) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3773 CrossRef; (c) R. Sheldon, Chem. Commun., 2001, 2399 RSC; (d) J. S. Wilkes, Green Chem., 2002, 4, 73 RSC.
- (a) B. C. Ranu and S. Banerjee, Org. Lett., 2005, 7, 3049 CrossRef CAS PubMed; (b) B. C. Ranu, A. Saha and S. Banerjee, Eur. J. Org. Chem., 2008, 519 CrossRef CAS; (c) B. C. Ranu, L. Adak and S. Banerjee, Tetrahedron Lett., 2008, 49, 4613 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: plausible mechanism for the synthesis of benzylpyrazolyl coumarins, reusability of [pmIm]Br, preparative method for the synthesis of benzylpyrazolyl coumarin derivatives, methods for the preparation of ionic liquids, copies of 1H NMR, 13C NMR spectra and HPLC data of benzylpyrazolyl coumarin derivatives. See DOI: 10.1039/c5ra16643h |
| This journal is © The Royal Society of Chemistry 2015 |