Hydrogen peroxide treated graphene as an effective nanosheet filler for separation application
Abstract
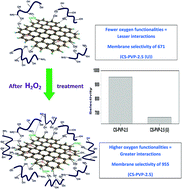
Graphene has been regarded as an important platform material to develop membranes for a wide range of applications. With state-of-the-art separation technologies, it is highly desirable to develop efficient membranes for organic dehydration that can reduce recovery costs. In this work, graphene loaded polymer composites of chitosan and poly(vinyl pyrrolidone) are used for pervaporation (PV) applications because the presence of a filler in the polymer matrix would boost membrane performance. Such an enhancement of barrier properties compared to conventional nascent membranes is a result of improved interface compatibility and interaction. In this research, H2O2 treated graphene was used as a nanofiller in a chitosan–poly(vinyl pyrrolidone) blend matrix to develop membranes that were tested for PV dehydration of ethanol as a function of filler loading, feed composition and temperature. Physico-chemical interactions between filler nanoparticles and the polymer matrix are responsible for the improved performance. The prepared membranes have been characterized via several analytical techniques. The 2.5 wt% H2O2 treated graphene loaded composite membrane offered a selectivity of 955, which is almost 40% higher than those membranes loaded with the same quantity of untreated fillers. Such enhanced membrane performance is attributed to an increase in the number of oxygen functionalities on the graphene surface after H2O2 treatment, resulting in improved filler interaction at the interface of the polymer and graphene in the presence of permeate molecules. Calculations involving the Flory–Huggins parameter, diffusion coefficient and Arrhenius activation energy barrier have been performed to explain the PV results in terms of the observed increase in membrane performance.

 Please wait while we load your content...
Please wait while we load your content...