Aminomethylpyrene-based imino-phenols as primary fluorescence switch-on sensors for Al3+ in solution and in Vero cells and their complexes as secondary recognition ensembles toward pyrophosphate†
Ajit Kumar Mahapatra*a,
Syed Samim Alia,
Kalipada Maitia,
Saikat Kumar Mannaa,
Rajkishor Majia,
Sanchita Mondala,
Md. Raihan Uddinb,
Sukhendu Mandalb and
Prithidipa Sahooc
aDepartment of Chemistry, Indian Institute of Engineering Science and Technology, Shibpur, Howrah-711103, West Bengal, India. E-mail: akmahapatra@rediffmail.com; Fax: +91 3326684564
bDepartment of Microbiology, University of Calcutta, Kolkata-700019, India
cDepartment of Chemistry, Visva-Bharati (A Central University), Santiniketan 731235, India
First published on 15th September 2015
Abstract
Three aminomethylpyrene-based salicyl-imines, viz. L1, L2 and L3 were synthesized and characterized and their recognition of biologically relevant Mn+ ions was studied. These three receptors were shown to be selective and sensitive for Al3+ among the 13 metal ions studied in a HEPES buffer medium by fluorescence, absorption, and visual emission color change with detection limits of 3.60, 2.13 and 2.16 μM, respectively, by L1, L2 and L3. The interaction of Al3+ with the three receptors (L1, L2 and L3) has been further supported by absorption studies, and the stoichiometry of the complex formed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) has been established on the basis of emission and ESI-MS. Competitive ion titrations carried out reveal that the Al3+ can be detected even in the presence of other metal ions of bio importance. The structure of the aluminium complexes and their mode of interactions were established by DFT calculations. TDDFT calculations were performed in order to demonstrate the electronic properties of receptors. Microstructural features of L2 and its Al3+ complex have been measured by AFM. Moreover, the utility of the receptors L1, L2 and L3 in showing aluminium recognition in live cells has also been demonstrated using Vero cells as monitored by fluorescence imaging. In situ prepared [AlL1] and [AlL3] complexes were found to be sensitive and selective toward phosphate-bearing ions and molecules and in particular to pyrophosphate (PPi) among the other 15 anions studied; however, [AlL2] was not sensitive toward any of the anions studied.
1) has been established on the basis of emission and ESI-MS. Competitive ion titrations carried out reveal that the Al3+ can be detected even in the presence of other metal ions of bio importance. The structure of the aluminium complexes and their mode of interactions were established by DFT calculations. TDDFT calculations were performed in order to demonstrate the electronic properties of receptors. Microstructural features of L2 and its Al3+ complex have been measured by AFM. Moreover, the utility of the receptors L1, L2 and L3 in showing aluminium recognition in live cells has also been demonstrated using Vero cells as monitored by fluorescence imaging. In situ prepared [AlL1] and [AlL3] complexes were found to be sensitive and selective toward phosphate-bearing ions and molecules and in particular to pyrophosphate (PPi) among the other 15 anions studied; however, [AlL2] was not sensitive toward any of the anions studied.
Introduction
The development of fluorescent sensors for ubiquitous cations and anions in nature has been a recent area of focus due to their potential applications in environmental detection, molecular catalysis, medicine and the monitoring of biological processes.1–3 In particular, the development of a fluorescent probe for aluminum ions in the presence of a variety of other metal ions has received great attention due to the widespread application of aluminum in modern life.4 However, excess aluminum can cause damage to certain human tissues and cells, resulting in diseases such as dementia and encephalopathy, Alzheimer's5 and Parkinson's diseases6 and are believed to be attributed to the toxicity of Al3+. Also, Al3+ causes neurofibrillary, enzymatic, and neurotransmitter changes in the central nervous system. Aluminum accumulation has been shown to cause cancer of the lung, breast, and bladder.7 Aluminum may also directly affect iron metabolism by influencing the absorption of iron via the intestine, hindering iron transport in the serum, and displacing iron by binding to transferring.8 The increase of Al3+ concentration in the environment due to acidic rain and human activities is deadly for growing plants.9 High concentration of Al3+ hampers plant performance,10 killing fish, algae, bacteria, and other species in aquatic ecosystems.11 The WHO recommended the average daily human intake of Al3+ of around 3–10 mg and weekly tolerable dietary intake as 7 mg kg−1 body weight.12 Therefore, the facile detection of Al3+ is crucial in environmental monitoring and biological assays, and remains a major requirement. However, compared to other transition metal ions, limited examples of fluorescence sensors based on small molecules for Al3+ have been reported.13Al3+ is also associated with various anions, especially with phosphates, owing to its strong chelation as well as bridging capability. Phosphate-based inorganic as well as organic molecules play fundamental roles in a wide range of chemical and biological processes of human life. Among the phosphates (Pi), inorganic pyrophosphate (PPi) is a particular interest due to an essential anion for normal cellular functioning and has numerous other applications in biology as well as in the environment.14 In addition, PPi has also been used as an additive in foods.15 The accumulation of the PPi complex of Ca2+ in the fluid of joints is indicative of the disease that is popularly known as calcium pyrophosphate deposition disease (CPDD).16 Therefore, the selective detection of PPi has been a major research focus. Although traditional methods of anion sensing such as the use of ion-selective electrodes have already been discovered, there is an increasing need to find alternative means of analysis, including the use of selective fluorescent chemosensors.17 In general, sensing of anions in aqueous system is much more challenging task than cation due to the strong hydration effects of anions. Recently, metal ion complexes have been used as receptors for phosphates, and this emerged as one of the most successful strategies, since it provides specific metal ion–anion interactions.18 In this regard, metal ion complexes are ideal binding sites for PPi recognition rather than hydrogen bonding interaction in aqueous or organo-aqueous solutions. So, the utilization of a metal ion complex as a binding site for PPi has been found to be the most successful strategy because the strong binding affinity between metal ions and PPi allows the detection of PPi in 100% aqueous solutions. To date, these studies frequently have adopted the fluorescent complexes containing a metal cation (such as Al3+) chelator coupled with a variety of chromophores.19
In our recent publication, we have demonstrated the selective detection of anions using C3-symmetry tri-arm 8-hydroxyquinoline based conjugates bearing metal ion ensemble.20 Therefore, it is of prime interest to develop chelating ligand-based molecular systems to provide better sensitivity and selectivity toward ions and molecules. Thus, in present paper, the synthesis and characterization of imino–phenolic–pyrene conjugates L1, L2 and L3, and their chemoensembles that formed upon binding of Al3+ to L1, L2 and L3, viz., [AlL1], [AlL2] and [AlL3], have been explored extensively to sense PPi selectively among common biological phosphates. The selection of salicyl-OH and imine moieties in the designed chemosensor are based on the considerations that it can function both as a fluorescent quencher and as the potential binding unit for metal ions.21 For a sensor based on the phenolic Schiff base molecular systems, fluorescence is quenched via owing to the isomerization of the imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond) as well as to the excited-state proton transfer (ESPT) from salicyl (–OH) to the imine nitrogen in the excited state. Upon complexation with a suitable metal ion, a large chelation-enhanced-fluorescence (CHEF) effect is observed. In the mean time, when a fluorophore contains an electron-donating group (often an amino group) e.g. L2 conjugating to a fluorophore, it undergoes ICT from the donor to the fluorophore upon light excitation. If a cation promotes the electron-donating character of the electron donor group, the absorption and fluorescence spectra should be red-shifted. The extended-conjugation enhanced the intramolecular charge transfer (ICT), which is expected to be highly sensitive towards external perturbations such as metal ion proximity resulting into optical and spectral changes. So, we expected that the combination of these two parts donor and binding moieties in common platform would generate a novel sensor with high selectivity and sensitivity for aluminium and PPi ions.
N bond) as well as to the excited-state proton transfer (ESPT) from salicyl (–OH) to the imine nitrogen in the excited state. Upon complexation with a suitable metal ion, a large chelation-enhanced-fluorescence (CHEF) effect is observed. In the mean time, when a fluorophore contains an electron-donating group (often an amino group) e.g. L2 conjugating to a fluorophore, it undergoes ICT from the donor to the fluorophore upon light excitation. If a cation promotes the electron-donating character of the electron donor group, the absorption and fluorescence spectra should be red-shifted. The extended-conjugation enhanced the intramolecular charge transfer (ICT), which is expected to be highly sensitive towards external perturbations such as metal ion proximity resulting into optical and spectral changes. So, we expected that the combination of these two parts donor and binding moieties in common platform would generate a novel sensor with high selectivity and sensitivity for aluminium and PPi ions.
Experimental section
General information and materials
The 1H and 13C NMR spectra were recorded on a Bruker AM-400 spectrometer using Me4Si as the internal standard. The 1H NMR chemical shift values are expressed in ppm (δ) relative to CHCl3 (δ = 7.26 ppm). Mass spectra were carried out using Water's QTOF Micro YA 263 mass spectrometer. UV-visible and fluorescence spectra measurements were performed on a SHIMADZU UV-1800 and a PerkinElmer LS-55 spectrofluorimeter respectively. DMSO of analytical grade was purchased from Spectrochem. All other materials for synthesis were purchased from Aldrich Chemical Co. and used without further purification. The solutions of anions were prepared from their tetrabutylammonium salts of analytical grade, and then subsequently diluted to prepare working solutions.Vero cells (very thin endothelial cell) (Vero 76, ATCC No CRL-1587) were used as models. Vero cells were incubated in PBS buffer (pH 7.4) containing 10 μM of the probe (L2) for 20 min at 37 °C, followed by washing the cells with the same buffer to remove the excess of the probes. At this stage, the fluorescence microscopy image of Vero cells displayed weak intracellular fluorescence. However, upon the addition of exogenous Al3+ into the cells for 20 min at 37 °C.
The AFM samples of L2, [L2 + Al3+] were prepared at 5 × 10−5 M concentration in ethanol. The receptor L2 was initially dissolved in 100 μL of CHCl3 and then made up with ethanol to the desired concentration. The stock solutions of these were sonicated for 20 min. Then 50–100 μL of aliquot was taken from this stock solution to spread over mica sheet using the drop cast method. The samples were then dried and analyzed by AFM technique.
Preparation of test solution for UV-vis and fluorescence study
A stock solution of the probe L1, L2 and L3 (2.0 × 10−5 M) were prepared in DMSO–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In titration experiments, each time a 2 × 10−5 M solution of L1, L2 and L3 were filled in a quartz optical cell of 1 cm optical path length, and the ion stock solutions were added into the quartz optical cell gradually by using a micropipette. Spectral data were recorded at 1 min after the addition of the ions. In selectivity experiments, the test samples were prepared by placing appropriate amounts of the ions (4 × 10−4 M) stock into 2 mL of solution of L (2 × 10−5 M).
1). In titration experiments, each time a 2 × 10−5 M solution of L1, L2 and L3 were filled in a quartz optical cell of 1 cm optical path length, and the ion stock solutions were added into the quartz optical cell gradually by using a micropipette. Spectral data were recorded at 1 min after the addition of the ions. In selectivity experiments, the test samples were prepared by placing appropriate amounts of the ions (4 × 10−4 M) stock into 2 mL of solution of L (2 × 10−5 M).
Computational studies
All geometries for L1, L2, L3 and [AlL1], [AlL2], [AlL3] were optimized by density functional theory (DFT) calculations using Gaussian 09 [B3LYP/6-31G(d)] software package.Synthesis and characterization of L1
The synthetic route of L1, L2 and L3 is shown in (Scheme 1). An ethanolic solution (3 mL) of pyrenemethylamine (250 mg, 1.0 mmol) was added to another ethanolic solution (3 mL) of salicylaldehyde (122.0 mg, 1.0 mmol). The mixed solution was refluxed for 4 h and then cooled to room temperature. A yellow precipitate was appeared, filtered, washed with EtOH for several times and then dried under vacuum. Then we got target product salicylaldehyde pyrenemethylamine Schiff-base (L1) which was dried under vacuum. The conjugate L2 and L3 were synthesized by adopting the similar procedure.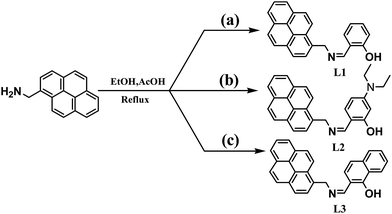 | ||
| Scheme 1 Synthesis of the probe L1, L2 and L3. Reagents: (a) salicylaldehyde; (b) 4-N,N-diethyl amino salicylaldehyde; (c) 1-hydroxy-2-naphthaldehyde. | ||
Results and discussion
The chemosensing ensembles were prepared by mixing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mol ratio of chemosensors L1/L2/L3 and Al3+ in the 1
2 mol ratio of chemosensors L1/L2/L3 and Al3+ in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v HEPES buffer–ethanol mixture at pH = 7.4 (referred to hereafter as “aqueous buffer solution), and these are used for all the studies with anions reported in this paper.
2 v/v HEPES buffer–ethanol mixture at pH = 7.4 (referred to hereafter as “aqueous buffer solution), and these are used for all the studies with anions reported in this paper.
Prior to being applied in the fluorescence sensing of anions, the binding interaction of chemosensors L1, L2 and L3 with metal ions were first studied by UV-vis absorption and fluorescence spectroscopy. The sensitivity of L1, L2 and L3 toward different metal ions and their preferential selectivity toward Al3+ over the other ions has been studied by fluorescence titrations.
The absorption spectra of free L1, L2 and L3 in aqueous DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; v/v) HEPES buffer (pH 7.4) exhibited different bands from 200 to 400 nm and these spectra are typical of a poly aromatic compound, the absorption arises mainly due to π–π* transitions of the signaling subunit.
2; v/v) HEPES buffer (pH 7.4) exhibited different bands from 200 to 400 nm and these spectra are typical of a poly aromatic compound, the absorption arises mainly due to π–π* transitions of the signaling subunit.
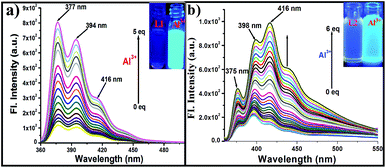
Upon the addition of Al3+ to a solution of L1, L2 and L3 separately in DMSO–water brought changes absorption spectra with common three absorption bands observed at ∼278, ∼330 and ∼350 nm. When L1 (Fig. 1b) and L2 (Fig. 1a) were titrated against Al3+ (0–10 equiv.), a significant decrease in the bands at 278, 330 and 350 nm and a new band was observed at ∼383 nm in case of L2, and its absorbance increases with observation of an isosbestic point at 372 nm. The band at ∼383 nm could be attributed to the intramolecular charge transfer transition from the donating diethylamino group to the imino-phenolic binding zone which acts as an acceptor during complexation, indicating the formation of the [AlL2] ensemble. The low energy (LE) band for L2 at 350 nm was gradually decreases, upon addition of Al3+ ions, which is responsible for the change of colour from yellow to colourless. This fact can be used for a ‘naked-eye’ detection of Al3+ ions.
The chemosensors L1, L2 and L3 exhibit very weak emission in the range 350–500 nm when excited at 330, 351 and 330 nm respectively. All of the studies were carried out in DMSO–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture at pH 7.4 to have an effective HEPES buffer concentration of 0.4 mM by maintaining the ligand concentration of 20 μM throughout the experiment and varying the mole ratio of the added metal ion. Titration of L1 (Fig. 2a), L2 (Fig. 2b) and L3 (Fig. S19†) with Al3+ results in the enhancement of fluorescence intensity as a function of the added Al3+ concentration.
1) mixture at pH 7.4 to have an effective HEPES buffer concentration of 0.4 mM by maintaining the ligand concentration of 20 μM throughout the experiment and varying the mole ratio of the added metal ion. Titration of L1 (Fig. 2a), L2 (Fig. 2b) and L3 (Fig. S19†) with Al3+ results in the enhancement of fluorescence intensity as a function of the added Al3+ concentration.
However, upon the progressive additions of Al3+ (0–5 equiv.) to the solution of L1 distinct fluorescent enhancement with dual emission bands centred at 377 and 394 nm were observed and a third weak band was observed at ∼416 nm (Fig. 2a). On the other hand L2 also showed dual emission bands at 375 and 398 nm upon similar additions of Al3+ along with a strong third emission band at 416 nm was observed (Fig. 2b). As for L3, it exhibits similar fluorescence responses as good as for L1 during the interaction with Al3+ (Fig. S19†).
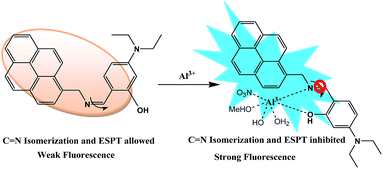
This fluorescence enhancement could be explained by an excited state intramolecular proton transfer (ESIPT) mechanism, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, and chelation enhanced fluorescence (CHEF) (Scheme 2).
N isomerization, and chelation enhanced fluorescence (CHEF) (Scheme 2).
When chemosensors L1, L2 and L3 exists as an unbound form, they exhibit weak fluorescence owing to the isomerization of the imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond) as well as to the excited-state intramolecular proton transfer (ESIPT) from salicyl-OH to the imine nitrogen.22 In the excited state, which has been well documented in the literature in the case of Schiff base molecular systems.23 By contrast, upon addition of Al3+ to chemosensors, a stable chelation by Al3+ to the imine nitrogen and phenolic oxygen brings rigidity to the conjugates and generating efficient chelation enhanced fluorescence (CHEF).24 Plot of relative fluorescence intensity (I/I0) versus [Al3+]/[L] mole ratio suggests the formation of a 1
N bond) as well as to the excited-state intramolecular proton transfer (ESIPT) from salicyl-OH to the imine nitrogen.22 In the excited state, which has been well documented in the literature in the case of Schiff base molecular systems.23 By contrast, upon addition of Al3+ to chemosensors, a stable chelation by Al3+ to the imine nitrogen and phenolic oxygen brings rigidity to the conjugates and generating efficient chelation enhanced fluorescence (CHEF).24 Plot of relative fluorescence intensity (I/I0) versus [Al3+]/[L] mole ratio suggests the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex in each case and hence is stoichiometric.
1 complex in each case and hence is stoichiometric.
The binding affinities of Al3+ toward L1, L2 and L3 have been calculated from the Benesi–Hildebrand equation and found to have binding constants 2.0 × 104 M, 4.1 × 104 M and 1.9 × 104 M (Fig. S15–S17 respectively and Table S1†) respectively. Higher binding constant observed in the case of L2 as compared to L1 and L3 speaks for the stronger binding of Al3+ with L2, as explained on the basis of the tight coordination due to enhanced intramolecular charge transfer (ICT) from electron donor diethylamino group to binding zone.
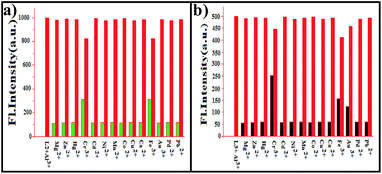
In order to check whether L1, L2 and L3 are sensitive to only Al3+ or even to the other ions, competitive titrations were carried out in the presence of other biologically and ecologically relevant metal ions. In the fluorescence titration, the result found, none of these metal ions significantly affect the emission intensities of L1 (Fig. S21†), L2 (Fig. 3a) and L3 (Fig. 4b) upon the addition of Al3+, to have only marginal changes in the emission intensity, suggesting that none of these ions interfere in the fluorescence emission of the Al3+ complex. Therefore, it can be concluded that L1, L2 and L3 recognizes Al3+ even in the presence of other metal ions. Fluorescence titrations were carried out in the same medium for L2 with 12 different metal ions, viz. Mg2+, Cu2+, Zn2+, Ni2+, Co2+, Fe3+, Ca2+, Hg2+, Cd2+, Au3+, Mn2+, Cr3+, found no significant fluorescence enhancement except Au3+, Cr3+ and Fe3+ revealed relatively insignificant fluorescence ON responses in this region (Fig. 4). This might be due to their paramagnetic properties which promote dissipation of the excited state energy in a non-radiative process as a result of spin–orbital coupling.25
Therefore, L1, L2 and L3 can be used for the selective recognition of Al3+ among the 12 different metal ions studied. Minimum detectable concentrations of L1, L2 and L3 are 3.6 μM, 2.01 μM and 2.16 μM (Fig. S11–S13 respectively and Table S1†) respectively, has been detected by the fluorescence titrations carried out by keeping the receptor to Al3+ mole ratio as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
To be useful in biological applications, it is necessary for a fluorescent probe to operate over a suitable range of pH, especially at physiological pH. A series of buffers with pH values ranging from 1 to 13 was prepared by mixing sodium hydroxide solution and hydrochloric acid in HEPES buffer.
Thus, we proceeded to investigate the effect of pH on the fluorescence intensity of the probe L1 (Fig. 5b) in absence or presence of Al3+. The results showed that fluorescence intensity (I377 nm) of L1 showed no apparent changes in the pH range from 1.0 to 13.0 no matter with or without Al3+, indicating that L1 is stable and its response towards Al3+ was also almost invariable in this pH range. Similar effect of pH was observed in case of L2 (Fig. S18†) and L3 (Fig. 5a).
ESI mass spectra obtained for in situ complexes of L1, L2 and L3 with Al3+ resulted in molecular ion peaks at (m/z) 536.1729 (calc. 536.1713), 563.0363 (calc. 564.2639) and 541.1962 (calc. 541.1964) respectively (Fig. S3, S7, and S10† respectively). The mass spectra are assignable to the mass of [L1 + Al + (NO3)2 + MeOH + H2O]+, [L2 + Al + (NO3) + MeOH + H2O + OH]+ and [L3 + Al + NO3 + MeOH + H2O + OH]+ supports the presence of aluminium. Therefore, we suggest that probes L1, L2 and L3 coordinates with Al3+ with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry.
1 stoichiometry.
In addition, we investigated the 1H NMR spectra of L2 in the presence of Al3+. The addition reaction of Al3+ to L2 was confirmed by the comparison of 1H NMR spectra of L2 with and without the addition of Al3+ in DMSO-d6 (Fig. 6).
 | ||
| Fig. 6 1H NMR chart (400 MHz, DMSO-d6, 0.5 mL) of L2 (10 mg) measured with different equivalent of Al3+. | ||
From NMR titration it is found that the peak corresponding to phenolic-OH proton gets broadened and both the phenolic-OH and imine-CH at 13.76 ppm and 8.56 ppm respectively get shifted downfield with the gradual addition of Al3+ ion. This clearly indicates that Al3+ is being coordinately bound with both phenolic oxygen and imine nitrogen atom.
To verify the structural features of the probes L1, L2 and L3, their aluminium complexes [AlL1], [AlL2], [AlL3] were addressed by computational studies.28,29 The geometry optimizations for probes (L1, L2 and L3) (Fig. S28a, S26a, and S30a†) and their complexes (Fig. 7) were done in a cascade fashion starting from semiempirical PM2 followed by ab initio HF to DFT B3LYP by using various basis sets, viz., PM2 → HF/STO-3G → HF/3-21G → HF/6-31G → B3LYP/6-31G(d). For optimization of the complexes, a starting model was generated by taking the DFT optimized probes (L1, L2 and L3) and placing the Al3+ ion well in the core of the pyrene enamine nitrogen and phenolic (–OH) as donors moieties at a non-interacting distance. The optimized complexes were mainly formed through the interaction of imine nitrogen and phenolic oxygen atom of probes (L1, L2 or L3) leads to distorted geometries by exhibiting coordination bond length in the range with N1–Al = 1.90 Å and O1–Al = 2.10 Å (for L1), N2–Al = 1.92 Å and O2–Al = 2.14 Å (for L2), N3–Al = 1.91 Å and O3–Al = 2.09 Å (for L3), (Fig. S28b, S26b, and S30b†).
The spatial distributions and orbital energies of HOMO and LUMO of probes (L1, L2 and L3) and their corresponding aluminium complexes [AlL1], [AlL2] and [AlL3] were also determined to explain the change of electronic properties of these complexes in their ground and excited states. The vertical transitions calculated by TDDFT (Tables S5, S3, S7,†) were compared with the UV-vis spectra of [AlL1], [AlL2] and [AlL3] were found to have good agreement with the experimental data.
The TDDFT calculations performed at the B3LYP/6-31G(d) level of theory revealed the absorption bands in the region of λmax 250–400 nm. These study suggest that the vertical transitions observed at ∼344 and ∼331 nm, ∼372 and ∼350 nm, ∼330 nm and ∼345 are comparable with those from experimental data for [AlL1], [AlL2] and [AlL3] respectively. In all cases, the highest observed oscillator strengths (F) correspond to the experimental λmax (at ∼351, ∼347 and ∼331 nm), that results from π → π* of the salicylimine and pyrene fragments of the probes. The π electrons on the relevant HOMOs and LUMOs of [AlL1], [AlL2] and [AlL3] complex are essentially distributed in the entire salicylimine and pyrene backbone respectively. By contrast, in the case of L1, the π-electrons on HOMO and HOMO−1 are primarily resided on the π-conjugated pyrene moiety. Corresponding molecular orbitals (MOs) of the three complexes for one electron excitations are shown in (Fig. S29, S27, S31†). Moreover, the HOMO–LUMO energy gaps of complexes [AlL1], [AlL2] and [AlL3] becomes much smaller relative to their probes L1, L2 and L3. The energy gaps between HOMO and LUMO in the probes and their complexes were shown in the ESI portion (Tables S4, S2 and S6†).
In order to understand how the microscopic structural features of the probe L2 changes from its in situ complexes [AlL2], the atomic force microscopy (AFM) technique was used. L1, L2, and L3 exhibit very similar recognition properties towards Al3+. Hence it was postulated that the microstructure of a representative sample [AlL2] would provide a reliable indication of the change in physical properties experienced by L1 and L3 due to interaction with Al3+.
The AFM images of the probes L2 and its complexes show that the particles were well spread over the mica sheet. The morphological feature of L2 is quite different from their respective complexes. While complexes forms large lumps, like aggregates of size 150 to 240 nm, such aggregation is not present in the probes L2 only. However, the probes L2 is uniformly distributed over the mica sheet with an average size of 90 to 140 nm. The formation of larger particles in [AlL2] is attributable to the Al3+ induced agglomerations of the probes (Fig. 8).
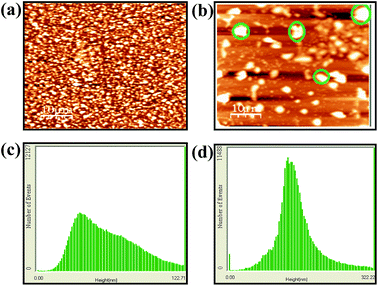 | ||
| Fig. 8 AFM micrographs of (a) L2 and (b) [Al3+ − L2]. Few representative lumps are encircled on (b). RMS roughness observed in AFM for L2 in the absence (c) and in the presence (d) of Al3+ ion. | ||
Owing to the strong affinity of Al3+ toward the phosphate, the highly switch on fluorescence with large fold enhancement of Al3+ complexes [AlL1] and [AlL3] have been studied for their secondary sensing property toward phosphate-based anions. Therefore, the in situ prepared aluminium complexes in aqueous buffer solution have been used as chemo-sensing ensembles for anions.
The recognition behavior of the in situ prepared ensemble Al3+ complexes [AlL1] and [AlL3] have been evaluated by carrying out the fluorescence titrations of [AlL1] and [AlL3] in aqueous buffer solution at pH 7.4 with different anions, viz., F−, Cl−, Br−, I−, NO3−, SO42−, AcO−, HSO4−, H2PO4−, (Pi), P2O74−, (PPi) as well as nucleotides. Fluorescence of [AlL3] has been quenched gradually in the case of inorganic pyrophosphate (PPi), and the complete quenching was observed at ∼10 equivalent owing to its strong binding nature toward Al3+. The fluorescence intensity of [AlL3] complex was decreases regularly with increasing concentration of PPi as shown in the inset of (Fig. 9a). Unlike the strong fluorescence decrease observed upon the addition of PPi, the other phosphate based anions such as Pi showed moderate fluorescence changes that too at higher mole ratios. This clearly suggests that the [AlL1] (Fig. S33†) and [AlL3] (Fig. 9b) are sensitive chemo-sensing ensemble for phosphate based anions, particularly, to the PPi, among the different anions studied in aqueous buffer solution (Fig. 9b). The selectivity for PPi over other anions can be attributed to a strong coordination of Al3+ to the phosphate unit, which was also explained by Yoon and Hong.26
The visual color changes from blue fluorescent to nonfluorescent in the presence of PPi that is similar to that of the L3, while all the other anions show no change in the color (Fig. 9a inset). The sensitivity of [AlL3] for PPi has been evaluated by measuring the detectable lowest concentration. The fluorescence titration carried out between [AlL3] and [PPi] by maintaining a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio resulted in 6.4 μM of PPi as the lowest detectable concentration in aqueous buffer medium (Fig. S15†). The [AlL2], which is contained of one electron donating NEt2 moiety in the phenyl ring as compared to [AlL3], does not show any significant fluorescence change toward any of these anion; however, [AlL2] shows small quenching of fluorescence intensity in the case of almost all the phosphate ions due to electron donating character of –NEt2 group increases electron density in the binding core. This is attributable to the binding of phosphate due to interaction between PPi and [AlL3] followed by the removal of Al3+ in the form of [Al·PPi], thus releasing the free L3. Thus the [AlL3] complex acts as a secondary recognition ensemble toward PPi. Removal of Al3+ bound to the Schiff's base core by the phosphate has been reported in the literature.27
1 ratio resulted in 6.4 μM of PPi as the lowest detectable concentration in aqueous buffer medium (Fig. S15†). The [AlL2], which is contained of one electron donating NEt2 moiety in the phenyl ring as compared to [AlL3], does not show any significant fluorescence change toward any of these anion; however, [AlL2] shows small quenching of fluorescence intensity in the case of almost all the phosphate ions due to electron donating character of –NEt2 group increases electron density in the binding core. This is attributable to the binding of phosphate due to interaction between PPi and [AlL3] followed by the removal of Al3+ in the form of [Al·PPi], thus releasing the free L3. Thus the [AlL3] complex acts as a secondary recognition ensemble toward PPi. Removal of Al3+ bound to the Schiff's base core by the phosphate has been reported in the literature.27
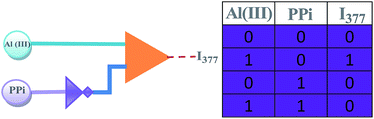
In general, molecules can undergo changes in the ground or excited states, in response to modulators which can be guest molecules, ions, or light of a certain wavelength. In most cases, these changes could then be signaled by changes in the emission intensity, or wavelength shift. Due to similar sensing behaviours of L1 and L3, it is safe to assert that the logic functions characteristic of one of the chemosensors will be faithfully reproduced by the other. So, the fluorescence result describe above for L1 by monitoring one output at 377 or 394 nm, we used our system for the construction of logic gate INHIBIT (INH) logic functions by using two sets of input signals, while one is Al3+ and the other is PPi. Without any chemical input, free compound L1 did not show any strong emissions at 377 nm. With respect to inputs, the presence and absence of Al3+ and PPi are defined as ‘‘1’’ and ‘‘0’’, respectively. Additionally, we define the fluorescence high and low signal as outputs ‘‘1’’ and ‘‘0’’, respectively. From the logic gate functions, the emission of L1 is observed only in the presence of single input, viz., Al3+ and not with PPi and Pi as these phosphates are insensitive to L1. This indicates that L1 is switched on only in the presence of Al3+ at 377 nm.
Similarly, in the presence of PPi fluorescence emission of [AlL1] was quenched and no significant output signal was observed. From these studies, it has been found that L1 can be used as INH logic gate toward Al3+ in the absence of PPi by observing emission at 377 nm. The two-input Al3+ and PPi mimics the performance of INH logic gate by observing emission at 377 nm. The truth table and the pictorial representation for the corresponding INH logic gate are given in Fig. 10.
The reversibility is an important aspect of any receptor to be employed as a chemical sensor for detection of specific metal ions. Both L1 and L3 showed similar behavior towards PPi, it is safe to assert that the reusability and reversibility of one of the chemosensors will be faithfully reproduced by the other.
So, the reusability of the probe L1 for sensing Al3+ has been demonstrated by carrying out four alternative cycles of the titration of L1 with Al3+ followed by PPi. Al3+ shows remarkable switch-on fluorescence changes through formation of [AlL1], and further the titration of this fluorescent complex with PPi quenches the same by removing the Al3+ from [AlL1] in DMSO–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) in HEPES buffer (20 mM, pH 7.4) (Fig. 11). The bright bluish fluorescence is immediately turned off. Titration of [AlL1] with PPi shows significant switch off fluorescence, and the fluorescence is regained when Al3+ is added to result in the switch on mode. Hence, L1 is a reversible and reusable sensor for Al3+ and its complex [AlL1] as a secondary sensor for PPi.
1, v/v) in HEPES buffer (20 mM, pH 7.4) (Fig. 11). The bright bluish fluorescence is immediately turned off. Titration of [AlL1] with PPi shows significant switch off fluorescence, and the fluorescence is regained when Al3+ is added to result in the switch on mode. Hence, L1 is a reversible and reusable sensor for Al3+ and its complex [AlL1] as a secondary sensor for PPi.
 | ||
| Fig. 11 Fluorescence experiment showing on–off reversible visual fluorescence color changes of L1 after each addition of Al3+ and PPi sequentially. | ||
To further demonstrate the practical application of the probes, we carried out experiments in living cells. In vitro studies established the ability of L2 to detect Al3+ in biological system with excellent selectivity. L2 is an obvious choice for cellular imaging study due to the presence of the electron donating NEt2 moiety which helps achieve the strongest analyte binding in case of L2 among the three sensors studied. This is evident from the binding constants [2 × 104 (M), 4.1 × 104 (M), 1.9 × 104 (M) for L1, L2, L3 respectively]. Vero cells (very thin endothelial cell) (Vero 76, ATCC No CRL-1587) were used as models (Experimental section). However, to materialize this objective it is a prerequisite to assess the cytotoxic effect of probe L2, Al3+ and the complex on live cells.
The well-established MTT assay (Fig. S32†), which is based on mitochondrial dehydrogenase activity of viable cells were adopted to study cytotoxicity of above mentioned compounds at varying concentrations mentioned in method section. Cytotoxicity measurements for each experiment shows that probes L2 does not exert any adverse effect on cell viability, same are the cases when cells were treated with varying concentrations of Al3+. Now, Vero cells were incubated in PBS buffer (pH 7.4) containing 10 μM of the probe L2 for 20 min at 37 °C, followed by washing the cells with the same buffer to remove the excess of the probes. At this stage, the fluorescence microscopy image of Vero cells displayed weak intracellular fluorescence. However, upon the addition of exogenous Al3+ into the cells for 20 min at 37 °C, the cells exhibited highly intense blue fluorescence (Fig. 12d). The control experiments carried out with Al3+ solution alone do not show any fluorescence (Fig. 12b). Thus, Vero cells incubated with L2 in the presence of Al3+ showed much greater fluorescence emission as compared to the cells which were not incubated. With this, suggesting that Al3+ is responsible for enhancing the fluorescence of L2 in the cells, as it has also been shown to exhibit fluorescence enhancement in the solution medium. These results clearly indicate that the imino–phenolic–pyrene probe L2 is effective intracellular Al3+ imaging agents with cell permeability.
Conclusion
The imino–phenolic–pyrene probes of L1, L2 and L3 have been synthesized and characterized. These were found to be sensitive and selective receptors for Al3+ in HEPES buffer medium. Their selectivity and sensitivity were demonstrated on the basis of fluorescence, absorption, 1H NMR spectroscopy, ESI mass spectrometry, and visual fluorescent color changes. L1, L2 and L3 can detect Al3+ up to 3.6 μM, 2.13 μM, 2.16 μM respectively by switch-on fluorescence, suggesting its applicability to detect Al3+ ions in aqueous HEPES buffer medium. TDDFT calculations were performed to demonstrate the electronic properties of L1, L2, L3 and their corresponding aluminium complexes. We have found significant similarities with the experimental results. In solution, [AlL2] shows strong fluorescence emission at 416 nm when excited at 351 nm through exhibition of an intense blue color fluorescence under UV light. To know the supramolecular microstructural features of L2 and [AlL2] complex, AFM studies were carried out in which the discrete shaped particles of L2 was found aggregated form in the complex. L2 was demonstrated as potential live-cell fluorescence imaging agents under microscopy using Vero cells. Weak fluorescent images were observed when Vero cells were incubated with these probes alone. However, strong fluorescence was observed in Vero cells in the presence of Al3+. Hence, these result clearly indicate that the imino–phenolic–pyrene probe, viz., L2, is effective intracellular Al3+ imaging agents with cell permeability. The in situ prepared fluorescent chemo-ensembles aluminium complexes, viz., [AlL1] and [AlL3], have been subjected to studies of their secondary sensing properties toward various anions. Owing to this unique features of these complexes have been used as a chemo-ensemble sensor for phosphate based anions in general and PPi in particular among the twelve different anions. These observed fluorescence responses at appropriate monitoring wavelengths could be used as a output signals to demonstrate in principle a basic INH logic gate properties using two inputs. These results will be useful for further molecular design to mimic the function of complex logic gates.Acknowledgements
We thank the DST-New Delhi [Project file no. SR/S1/OC-44/2012] for financial support. SM thanks to the UGC, New Delhi for a fellowship.Notes and references
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120 RSC.
- Z. Xu, X. Chen, H. N. Kim and J. Yoon, Chem. Soc. Rev., 2010, 39, 127 RSC.
- C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520 RSC.
- (a) H. G. Lee, J. H. Lee, S. P. Jang, I. H. Hwang, S. J. Kim, Y. Kim, C. Kim and R. G. Harriso, Inorg. Chim. Acta, 2013, 394, 542 CrossRef CAS PubMed; (b) J. H. Kim, J. Y. Noh, I. H. Hwang, J. Kang, J. Kim and C. Kim, Tetrahedron Lett., 2013, 54, 2415 CrossRef CAS PubMed; (c) J. Kang, H. K. Kang, H. Kim, J. Lee, E. J. Song, K. D. Jeong, C. Kim and J. Kim, Supramol. Chem., 2013, 25, 65 CrossRef CAS PubMed; (d) H. Y. Lin, P. Y. Cheng, C. F. Wan and A. T. Wu, Analyst, 2012, 137, 4415 RSC.
- D. P. Perl and A. R. Brody, Science, 1980, 208, 297 CAS.
- Z. Dai, N. Khosla and J. W. Canary, Supramol. Chem., 2009, 21, 296 CrossRef CAS PubMed.
- (a) B. Armstrong, C. Tremblay, D. Baris and G. Theriault, Am. J. Epidemiol., 1994, 139, 250 CAS; (b) P. D. Darbre, J. Inorg. Biochem., 2005, 99, 1912 CrossRef CAS PubMed.
- C. Exley, L. Swarbrick, R. K. Gherardi and F. J. Authier, Med. Hypotheses, 2009, 72, 135 CrossRef CAS PubMed.
- A. B. S. Poleo, Environ. Pollut., 1997, 96, 129 CrossRef CAS.
- (a) E. Àlvarez, M. L. Fernández-Marcos, C. Monterroso and M. J. Fernández-Sanjurjo, For. Ecol. Manage., 2005, 211, 227 CrossRef PubMed; (b) J. Barcelo and C. Poschenrieder, Environ. Exp. Bot., 2002, 48, 75 CrossRef CAS.
- (a) N. E. W. Alstad, B. M. Kjelsberg, L. A. Vøllestad, E. Lydersen and A. B. S. Poléo, Environ. Pollut., 2005, 133, 333 CrossRef CAS PubMed; (b) E. Delhaize and P. R. Ryan, Plant Physiol., 1995, 107, 315 CAS; (c) J. Ren and H. Tian, Sensors, 2007, 7, 3166 CrossRef CAS PubMed.
- (a) J. Barcelo and C. Poschenrieder, Environ. Exp. Bot., 2002, 48, 75 CrossRef CAS; (b) B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3 CrossRef CAS; (c) Z. Krejpcio and R. W. P. J. Wojciak, Environ. Stud., 2002, 11, 251 CAS.
- (a) S. Guha, S. Lohar, A. Sahana, A. Banerjee, D. A. Safin, M. G. Babashkina, M. P. Mitoraj, M. L. Bolte, Y. Garcia, S. K. Mukhopadhyay and D. Das, Dalton Trans., 2013, 42, 10198 RSC; (b) M. Mukherjee, S. Pal, S. Lohar, B. Sen, S. Sen, S. Banerjee, S. Banerjee and P. Chattopadhyay, Analyst, 2014, 139, 4828 RSC; (c) Z. Wang, M. A. Palacios and P. Anzenbacher, Jr., Anal. Chem., 2008, 80, 7451 CrossRef CAS PubMed; (d) H. Shirase, Y. Mori, Y. Fukuda and M. Uchiyama, Monatsh. Chem., 2009, 140, 801 CrossRef CAS.
- (a) D. C. Silcox and D. J. J. McCarty, Clin. Invest., 1973, 52, 1863 CrossRef CAS PubMed; (b) M. Ronaghi, S. Karamohamed, B. Pettersson, M. Uhlen and P. Nyren, Anal. Biochem., 1996, 242, 84 CrossRef CAS PubMed; (c) S. K. Kim, D. H. Lee, J. N. Hong and J. Yoon, Acc. Chem. Res., 2009, 42, 23 CrossRef CAS PubMed.
- (a) C. P. Li, H. R. Ibrahim, Y. Sugimoto, H. Hatta and T. J. Aoki, J. Agric. Food Chem., 2004, 52, 5752 CrossRef CAS PubMed; (b) P. K. Datta, A. C. Frazer, M. Sharratt and H. G. Sammons, J. Sci. Food Agric., 1962, 13, 556 CrossRef CAS PubMed.
- (a) C. Beck, H. Morbach, M. Stenzel, H. Collmann, P. Schneider and H. J. Girschick, Open Bone J., 2009, 1, 8 CrossRef CAS; (b) M. Doherty, C. Becher, M. Regan and A. J. Ledingham, Ann. Rheum. Dis., 1996, 66, 432 CrossRef; (c) A. E. Timms, Y. Zhang, R. G. Russell and M. A. Brown, Rheumatology, 2002, 41, 725 CrossRef CAS PubMed.
- (a) S. K. Kim, D. H. Lee, J. I. Hong and J. Yoon, Acc. Chem. Res., 2009, 42, 23 CrossRef CAS PubMed; (b) S. Anbu, S. Kamalraj, C. Jayabaskaran and P. S. Mukherjee, Inorg. Chem., 2013, 52, 8294 CrossRef CAS PubMed; (c) T. Sakamoto, A. Ojida and I. Hamachi, Chem. Commun., 2009, 141 RSC; (d) T. Cheng, T. Wang, W. Zhu, X. Chen, Y. Yang, Y. Xu and X. Qian, Org. Lett., 2011, 13, 3656 CrossRef CAS PubMed.
- (a) J. R. Lakowicz, Principle of fluorescence spectroscopy, 3rd edn, 2006 Search PubMed; (b) A. P. de Silva, H. Q. N. Gunaratne, T. A. Gunnlaugsson, T. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef CAS PubMed; (c) B. Valeur, J. Bourson, J. Pouget and A. W. Czarnik, Fluorescent Chemosensors for Ion and Molecule Recognition; ACS Symposium Series 538, American Chemical Society, Washington, DC, 1993 Search PubMed.
- (a) A. Sahana, A. Banerjee, S. Lohar, S. Das, I. Hauli, S. K. Mukhopadhyay, J. S.Matalobos and D. Das, Inorg. Chim. Acta, 2013, 398, 64 CrossRef CAS PubMed; (b) Y. Mia, Z. Caob, Y. Chena, S. Longb, Q. Xiea, D. Lianga, Z. Wenpinga and J. Xianga, Sens. Actuators, B, 2014, 192, 164 CrossRef PubMed; (c) W. H. Dinga, D. Wanga, X. J. Zhenga, W. J. Dingb, J. Q. Zhengc, W. H. Mud, W. Caoa and L. P. Jin, Sens. Actuators, B, 2015, 209, 359 CrossRef PubMed.
- A. K. Mahapatra, S. K. Manna, C. D. Mukhopadhyay and D. Manda, Sens. Actuators, B, 2014, 200, 123 CrossRef CAS PubMed.
- V. Kumar, A. Kumar, U. Diwan, Shweta, Ramesh, S. K. Srivastava and K. K. Upadhyay, Sens. Actuators, B, 2015, 207, 650 CrossRef CAS PubMed.
- (a) S. Ameer-Beg, S. M. Ormson, R. G. Brown, P. Matousek, M. Towrie, E. T. J. Nibbering, P. Foggi and F. V. R. Neuwahl, J. Phys. Chem. A, 2001, 105, 3709 CrossRef CAS; (b) A. Weller, Z. Elektrochem., 1956, 60, 1144 CAS; (c) W. Chen, Y. Xing and Y. Pang, Org. Lett., 2011, 13, 1362 CrossRef CAS PubMed; (d) S. Lim, J. Seo and S. Y. Park, J. Am. Chem. Soc., 2006, 128, 14542 CrossRef CAS PubMed.
- (a) D. Maity and T. Govindaraju, Eur. J. Inorg. Chem., 2011, 5479 CrossRef CAS PubMed; (b) J. Wu, W. Liu, X. Zhuang, F. Wang, P. Wang, S. Tao, X. Zhang, S. Wu and S. Lee, Org. Lett., 2007, 9, 33 CrossRef CAS PubMed; (c) Y. K. Jang, U. C. Nam, H. L. Kwon, I. H. Hwang and C. Kim, Dyes Pigm., 2013, 99, 6 CrossRef CAS PubMed.
- (a) S. Sen, T. Mukherjee, B. Chattopadhyay, A. Moirangthem, A. Basu, J. Marek and P. Chattopadhyay, Analyst, 2012, 137, 3975 RSC; (b) C. Chen, D. Liao, C. Wan and A. Wu, Analyst, 2013, 138, 2527 RSC.
- (a) M. Tarek, M. Zaki, L. F. M. Esmail, A. Y. El-Sayed and Z. Fresenius, Anal. Chem., 1988, 331, 607 CrossRef CAS; (b) B. Bodenant, F. Fages and M. Delville, J. Am. Chem. Soc., 1998, 120, 7511 CrossRef CAS.
- C. R. Lohani, J. M. Kim, S. Y. Chung, J. Yoon and K. H. Lee, Analyst, 2010, 135, 2079 RSC.
- M. Hosseini, M. R. Ganjali, M. Tavakoli, P. Norouzi, F. Faridbod, H. Goldooz and A. Badiei, J. Fluoresc., 2011, 21, 1509 CrossRef CAS PubMed.
- (a) A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS PubMed; (b) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS; (c) D. Andrae, U. Haeussermann, M. Dolg, H. Stoll and H. Preuss, Theor. Chim. Acta, 1990, 77, 123 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16641a |
| This journal is © The Royal Society of Chemistry 2015 |