Improved photocatalytic performance of self-assembled Bi/BiOBr square microflowers with square nanopetals†
Abstract
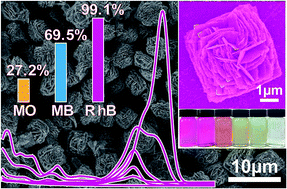
Herein we report the first example of the creation of Bi/BiOBr square microflowers with square nanopetals by a one-pot solvothermal process using ethylene glycol as both a solvent and a source of the reductant simultaneously. The square microflower material exhibited good photoelectric response and extremely high photodegradation efficiency for rhodamine B. We believe that this study represents an important advance regarding Bi-based inorganic materials.

 Please wait while we load your content...
Please wait while we load your content...