Synthesis of monodisperse copper nanoparticles using a modified digestive ripening technique and formation of superlattices†
A. H. Shaik and
J. Chakraborty*
Department of Chemical Engineering, Indian Institute of Technology Kharagpur, West Bengal, India. E-mail: jayanta@che.iitkgp.ernet.in; aabid.iitkgp@iitkgp.ac.in
First published on 6th October 2015
Abstract
In this work we demonstrate for the first time the synthesis of monodisperse copper nanoparticles (5 ± 0.9 nm) and formation of 2-D and 3-D superlattices. Superlattices have been formed using slow destabilization of the colloid. A confined environment of the emulsion droplet has been used to promote formation of 3D superlattices. It has been shown that very compact crystal like superlattices can be formed if the destabilized sol is aged for 10 days at −4 °C.
One of the emerging areas in nanotechnology is the synthesis of nanoparticle superlattices. Superlattices are well-defined ordered structures obtained by nanoparticle self assemblies.1 Superlattices have many important applications. For example, FePt nanoparticle superlattices can be used in data storage.2 Some nanocrystal superlattices find applications as magnetic materials with enhanced properties3,4 or in photovoltaics.5 Superlattices of gold find applications as biosensors.6 Gold nanoparticle superlattices can also be used as photoluminescent materials.7
In spite of these interesting applications, the synthesis of superlattices remains a less explored area. The main challenge in the formation of superlattices is the initial preparation of monodisperse nanoparticles with a COV of around 10%. Although it is possible to form superlattices from more polydispersed particles utilizing ligands (like DNA) that latch onto both particles,8 monodisperse particles are always desirable for superlattice formation.
A very few methods have been developed to synthesize such monodisperse nanoparticles. These include thermal decomposition of organometallic precursors9–11 and efficient post heat treatment technique such as digestive ripening,12–14 solution annealing15 or solid state heat treatment.16 Digestive ripening is a process in which polydisperse colloid is converted to monodisperse colloid by refluxing in presence of excess capping ligand. While digestive ripening and solution annealing produces particles over a narrow size range (∼5–7 nm), the solid state heat treatment method16 offer the advantage of size tuning from 1.5 to 9.7 nm. In this work, post heat treatment technique has been used to prepare monodisperse nanoparticles of copper.
Klabunde's group17 pioneered the synthesis of monodisperse gold nanoparticles by heat treatment technique and also formed superlattice from the uniform colloid. They name the method as ‘digestive ripening’ which is a well established method now for formation of uniform gold colloid. A number of studies have been conducted to explore various features of this method.13,14 On the other hand, much less number of investigations have been conducted on heat treatment of other metals. Digestive ripening and superlattice formation has been reported for indium18 and silver19 but synthesis of monodisperse copper nanoparticles using digestive ripening technique and formation of superlattice has not been reported so far. In this report, we demonstrate a modified digestive ripening technique for Cu and form 2-D and 3-D superlattices of Cu nanoparticles from the monodisperse nanoparticles.
Superlattices can be formed in a colloid by slowly evaporating the solvent from the colloid. In this process, the concentration of the particles gradually increases which is followed by nucleation of small particle clusters finally leading to the formation of superlattice.20,21 Demortiere et al. (2008)22 and Zhang et al. (2012)23 observed formation of 3-D superlattices of platinum nanocubes and Pt3Cu2 nanooctahedrons on TEM grid by slow evaporation of the solvent upon drying of the colloid.
Another way to form superlattice is to add an antisolvent to the colloid and thereby slightly destabilizing the colloid. However, the flock form by this method should remain suspended so that the re-arrangement of particles and compaction of superlattices can take place. If the newly formed superlattices are allowed to move freely in the destabilized solvent, they aggregate and settle at the bottom. To avoid such mutual aggregation, confined environment is needed. This can be achieved by using microchannel slug flow.24 Recently Dutta et al. (2014) used emulsion droplets to this effect.25 This emulsion technique has been used in the present study for superlattice formation.
Copper nanoparticles in toluene phase was synthesized by modifying the Brust's method. In this method, copper ions were transferred to the organic phase from aqueous phase using the ion transfer agent tetraoctylammonium bromide (TOAB) and then reduced using sodium borohydride in the presence of dodecane thiol. A detailed procedure is available in the ESI.†
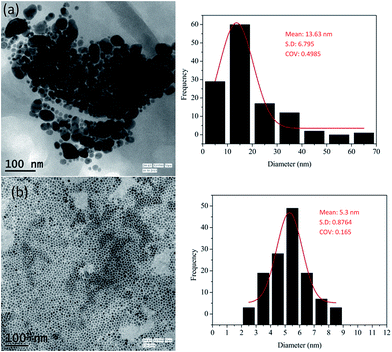
The particles produced by this method are polydisperse in nature (shown in Fig. 1(a)) and hence digestive ripening should be used to narrow down the size distribution. However, the usually reported digestive ripening process17 could not be used and a modified protocol has been used. The main deviation from the protocol reported in ref. 17 is that the precipitation and redispersion of the polydisperse colloid should be avoided to prevent the irreversible aggregation of particles. Also the ripening duration is much prolonged in compared to those usually reported. Scheme 1 represents the modified digestive ripening process. A detailed description of the process is available in the ESI.†
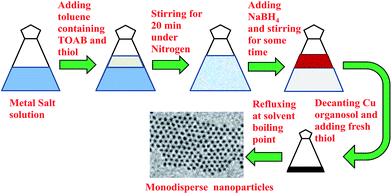 | ||
| Scheme 1 Schematic showing the modified digestive ripening process specially suitable for Cu nanoparticles. | ||
The particles produced before and after digestive ripening are shown in Fig. 1. The initial particles are highly polydispersed as shown in Fig. 1(a) with mean size of 13.6 nm and COV = 0.5. The monodisperse particles after 24 hours of ripening process are shown in Fig. 1(b). These particles are very uniform with a mean particle size of 5.3 nm and COV = 0.16.
Prolonged duration of ripening is very essential to form uniform Cu nanoparticles. While 1.5 h digestive ripening was sufficient for gold,12 much prolonged (∼20 h) ripening is needed for Cu. This has been confirmed by performing the digestive ripening for 3, 6, 15 and 24 h as shown in Fig. S1.† It has been observed that the particles remain polydisperse (COV = 0.22) at 6 h and 15 h (COV = 0.20) and approaches the final COV around 24 hours.
UV-Vis of the copper nanoparticles produced before and after digestive ripening are shown in Fig. S2.† It can be seen that while the characteristic peak of copper is present in the un-ripened sample, this peak is missing in the ripened sample. Hence, thorough characterization of the sample was needed. First, XRD analysis of the particles was conducted. The colloid was dried by nitrogen blowing and subsequent storage in vacuum desiccators. The waxy mass generated was analyzed in a diffractometer and the pattern obtained is shown in Fig. S3.† The XRD shows prominent peaks for Cu with two smaller peaks for its oxide. The crystallite size calculated from these peaks are small (4.5 nm) which is consistent with the TEM observations. It also shows a pair of strong peaks at 2θ = 14.287 and 2θ = 23.402 which correspond well with those of CuSR (JCPDS: 88-2139). The crystallite size corresponding to these peaks are much larger (71 nm). Since etching of metal is known to happen during digestive ripening,26 this phenomenon is expected. HRTEM of the samples also clearly suggests that the sample is a mixture of copper and its oxide (probably formed due to exposure to atmospheric oxygen during drying of the grid). The lattice fringes show a d-spacing of 0.21 nm and 0.25 nm from two pairs of spots in the FFT which corresponds to 111 face of Cu and 002 face of CuO respectively (Fig. S4†).
It may be concluded from the foregoing discussion that the particles are of copper with some amount of oxide impurity. We believe that the oxide impurity is very small in the colloid because the as synthesized colloid does not show any oxide peak. But if the samples are stored for prolonged period (more than a month) without proper sealing, the reddish brown colloid turns into green (picture shown in Fig. S5†). While the reddish brown colloid show the plasmonic feature as shown in Fig. S2,† the green colloid show a distinct peak at 680 nm consistent with copper oxide (Fig. S6†). The green colloid, when shaken with sodium borohydride solution, turns back into reddish brown colloid with usual UV-Vis feature as shown in Fig. S2.† Hence, the particles synthesized are Cu with small amount of oxide impurity.
Next, we form 3D superlattices using these monodisperse particles inside an emulsion droplet. Here, oil in water emulsion has been used as a confined environment. To form the oil phase, 100 μl 1-butanol was added to 400 μl of Cu colloid as destablizing agent. 1-Butanol modifies the Hamaker constant of the medium and thereby increases the attractive potential between particles slightly. Next, aqueous phase is prepared by adding 90 μl of Tween 80 to 4.41 ml of water. Later these two phases (0.5 ml destabilized Cu colloid and 4.5 ml surfactant solution) are mixed and stirred vigorously at 1400 rpm for about 10 minutes using a Teflon coated magnetic stirrer to make an emulsion. This emulsion is stored at different temperature for superlattice formation.
Other antisolvents like ethanol or propanol have also been tried but they promote rapid flocculation and hence porous flocks form. The concentration of 1-butanol has been optimised to control the flocculation process. It seems that 20% v/v 1-butanol in copper sol results optimally slow flocculation. These flocs remains suspended in solution for longer periods and form ordered structures.
The emulsions obtained were stored at different temperatures for certain period to form superlattices. Fig. 2 shows the TEM images of the top creamy layer of emulsion stored at 5 °C for 5 days. It can be seen that 3D superlattices have not grown yet but 2D superlattice over very large area similar to those observed by other researchers for gold nanoparticles is formed13 on the TEM grid.
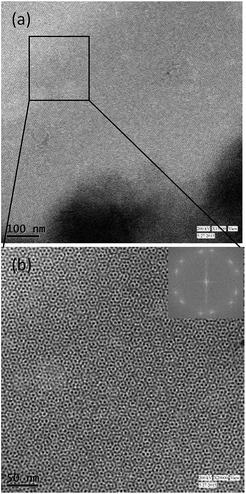 | ||
| Fig. 2 TEM images of copper nanoparticles stored in emulsion at 5 °C for 5 days (a and b) at different magnifications. Inset shows the corresponding FFT pattern. | ||
It has been observed previously,25 that lowering the temperature of storage is beneficial for the formation of 3D superlattices instead of increasing the amount of butanol. Hence, we store this emulsion at −4 °C for 5 days next. It can be observed that the 3D superlattice structure has not formed yet but large 2D superlattices are formed with variety of packing as shown in Fig. 3.
 | ||
| Fig. 3 TEM image of copper nanoparticles stored in emulsion at −4 °C for 5 days. Inset shows the corresponding FFT pattern. | ||
Additional aging of emulsion seems to be necessary to form crystal like close packed 3D structures. Very good 3-D superlattices of extremely compact and sharp edges are formed when the emulsion was stored for 10 days at −4 °C as shown in Fig. 4. We also store the emulsion at 5 °C for 10 days. In this case although 3-D superlattices are formed (Fig. S7†), but the compaction process seems to be incomplete.
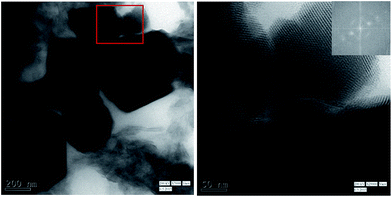 | ||
| Fig. 4 TEM images of copper nanoparticles stored in emulsion at −4 °C for 10 days ((a) (scale bar: 200 nm) and (b) (scale bar: 50 nm)) at different magnifications. Inset shows the corresponding FFT pattern indicating the FCC arrangement of particles similar to those observed by other researchers for gold.13 | ||
Conclusions
A method has been reported for formation of 3-D superlattices of copper nanoparticles. A well known post heat treatment technique (digestive ripening) has been successfully modified to produce uniform copper nanoparticles suitable for superlattice formation. In the modified method, the cleaning step has not been used and the digestive ripening has been carried out for 24 h instead of usual practice of 1.5 h used for gold. The superlattice has been formed in an emulsion droplet by slow destabilization method. Emulsion droplets have been used to prevent mutual aggregation of the superlattices. The aging period and the temperature of the destabilized sol have been found to be two key variables for superlattice formation. Aging for 10 days at −4 °C has been found to be the optimum condition required for formation of faceted 3D superlattices.Acknowledgements
We acknowledge financial support from SRIC (ISIRD Grant), IIT Kharagpur. We also acknowledge helpful discussions with Dr B. L. V. Prasad, National Chemical Laboratory, Pune, India. The authors also thank Mr Sudipto Mondal, Central Research Facility, IIT Kharagpur for help in TEM imaging.Notes and references
- K. Soulantica, A. Maisonnat, M.-C. Fromen, M.-J. Casanove and B. Chaudret, Angew. Chem., Int. Ed. Engl., 2003, 42, 1945–1949 CrossRef CAS PubMed.
- S. Sun, Adv. Mater., 2006, 18, 393–403 CrossRef CAS PubMed.
- L. Ca, M. Srtio, S. A. Fedoseev, A. V. Pan, S. Rubanov, I. A. Golovchanskiy and O. V. Shcherbakova, ACS Nano, 2013, 7, 286–293 CrossRef PubMed.
- H. Zhu, C. Xiao, H. Cheng, F. Grote, X. Zhang, T. Yao, Z. Li, C. Wang, S. Wei, Y. Lei and Y. Xie, Nat. Commun., 2014, 5, 3960 CAS.
- J. A. Labastide, M. Baghgar, I. Dujovne, Y. Yang, A. D. Dinsmore, B. G. Sumpter, D. Venkataraman and M. D. Barnes, J. Phys. Chem. Lett., 2011, 2, 3085–3091 CrossRef CAS.
- E. S. Shibu, M. A. Habeeb Muhammed, K. Kimura and T. Pradeep, Nano Res., 2009, 2, 220–234 CrossRef CAS.
- Z. Wang, L. Wu, W. Cai and Z. Jiang, J. Mater. Chem., 2012, 22, 3632–3636 RSC.
- R. J. Macfarlane, B. Lee, M. R. Jones, N. Harris, G. C. Schatz and C. A. Mirkin, Science, 2011, 334, 204–209 CrossRef CAS PubMed.
- J. Park, J. Joo, S. G. Kwon, Y. Jang and T. Hyeon, Angew. Chem., Int. Ed. Engl., 2007, 46, 4630–4660 CrossRef CAS PubMed.
- P. Jeevanandam, C. K. Srikanth and S. Dixit, Mater. Chem. Phys., 2010, 122, 402–407 CrossRef CAS PubMed.
- W. W. Yu, J. C. Falkner, C. T. Yavuz and V. L. Colvin, Chem. Commun., 2004, 2306–2307 RSC.
- B. L. V. Prasad, S. I. Stoeva, C. M. Sorensen and K. J. Klabunde, Chem. Mater., 2003, 15, 935–942 CrossRef CAS.
- P. Sahu and B. L. V. Prasad, Nanoscale, 2013, 5, 1768–1771 RSC.
- P. Sahu and B. L. V. Prasad, Langmuir, 2014, 30, 10143–10150 CrossRef CAS PubMed.
- M. M. Maye, W. Zheng, F. L. Leibowitz and N. K. Ly, Langmuir, 2000, 16, 490–497 CrossRef CAS.
- T. Shimizu, T. Teranishi, S. Hasegawa and M. Miyake, J. Phys. Chem. B, 2003, 107, 2719–2724 CrossRef CAS.
- S. Stoeva, K. J. Klabunde and C. M. Sorensen, J. Am. Chem. Soc., 2002, 124, 2305–2311 CrossRef CAS PubMed.
- S. Cingarapu, Z. Yang, C. M. Sorensen and K. J. Klabunde, Inorg. Chem., 2011, 50, 5000–5005 CrossRef CAS PubMed.
- A. B. Smetana, K. J. Klabunde and C. M. Sorensen, J. Colloid Interface Sci., 2005, 284, 521–526 CrossRef CAS PubMed.
- M. Wu, M. I. Bodnarchuk, M. V. Kovalenko, Ḱ. S. Pichler and G. Fritz-popovski, ACS Nano, 2010, 4, 423–431 CrossRef PubMed.
- D. K. Smith, B. Goodfellow, D. Smilgies and B. A. Korgel, J. Am. Chem. Soc., 2009, 131, 3281–3290 CrossRef CAS PubMed.
- A. Demortiere, P. Launois, N. Goubet, P. Albouy, C. Petit and P. Marie, J. Phys. Chem. B, 2008, 112, 14583–14592 CrossRef CAS PubMed.
- J. Zhang, Z. Luo, B. Martens, Z. Quan, A. Kumbhar, N. Porter, Y. Wang, D. Smilgies and J. Fang, J. Am. Chem. Soc., 2012, 134, 14043–14049 CrossRef CAS PubMed.
- M. I. Bodnarchuk, L. Li, A. Fok, S. Nachtergaele, R. F. Ismagilov and D. V. Talapin, J. Am. Chem. Soc., 2011, 133, 8956–8960 CrossRef CAS PubMed.
- A. Dutta, J. Chakraborty, B. L. V. Prasad and P. Sahu, J. Colloid Interface Sci., 2014, 420, 41–49 CrossRef CAS PubMed.
- S. S. Deepti and B. L. V. Prasad, New J. Chem., 2011, 35, 755–763 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and TEM, UV-Vis, HRTEM, XRD, SAED and SEM. See DOI: 10.1039/c5ra16508c |
| This journal is © The Royal Society of Chemistry 2015 |