Porous silicon nanoparticles as a nanophotocathode for photoelectrochemical water splitting†
Soundarrajan Chandrasekarana,
Steven J. P. McInnesa,
Thomas J. Macdonaldb,
Thomas Nann*b and
Nicolas H. Voelcker*a
aFuture Industries Institute, University of South Australia, Mawson Lakes Blvd, Adelaide, SA 5095, Australia. E-mail: Nico.Voelcker@unisa.edu.au
bIan Wark Research Institute, University of South Australia, Mawson Lakes Blvd, Adelaide, SA 5095, Australia. E-mail: Thomas.Nann@vuw.ac.nz
First published on 6th October 2015
Abstract
The antireflective properties and nanometer sizes of silicon nanoparticles can be exploited for improved solar energy conversion. We report on using porous silicon nanoparticles as a photocathode for photoelectrochemical water splitting. An enhancement in the photocurrent density was observed when porous silicon nanoparticles were decorated with indium phosphide nanocrystals and a bio-inspired iron sulfur carbonyl electrocatalyst. Our system gave a photocurrent density of −2.2 μA cm−2 while generating hydrogen gas.
Introduction
The generation of hydrogen (H2) through solar water electrolysis provides a clean and renewable source of energy.1–4 Photocatalytic and photoelectrochemical (PEC) water splitting are two ways of producing H2 from sunlight.1 For example, photocatalysts can absorb sunlight (of the appropriate energy) and split water molecules into H2 and oxygen (O2) when freely suspended in water.2,3,5–8 Silicon, with a band-gap of 1.12 eV is considered to be one of the most suitable materials used in photovoltaic devices, especially when compared to titanium dioxide (band gap 3.0 eV), nickel oxide (band gap 3.6 eV) cadmium selenides (band gap 1.7 eV) and cadmium sulphide (band gap 2.4 eV).9–12 Albeit silicon possesses low band-gap energy, by itself it is not sufficient to split water, which is why tandem-structures, such as semiconductor nanocrystals (NCs), are necessary. Bulk and nanostructured silicon materials in the form of planar silicon,13,14 porous silicon (pSi)15,16 and silicon nanowires (Si NWs)17–19 have been widely used for water splitting. Over the years, pSi nanoparticles (NPs) have been used as photoactive materials for chemical/photo-chemical H2 evolution.20–24 During the water splitting process, silicon hydride (Si–H) terminated pSi NPs undergo oxidation while reducing the protons to H2 in the electrolyte solution.20–24Previous reports show 10 nm sized pSi NPs synthesised using CO2 laser pyrolysis,20 achieve 100 times faster H2 production (g H2 per s per g silicon) than electrochemically etched pSi NPs of approximately 3–10 nm,21 8–20 nm (ref. 22) and 75 nm commercially purchased pSi NPs.23 However, recent research into H2 production was demonstrated from mesoporous silicon microparticles of approximately 5–20 μm, synthesised by reducing silicon halogenide.24 The authors claimed that the high surface area of those mesoporous silicon microparticles significantly improved the H2 generation. It was found that the H2 production (g H2 per s per g silicon) was 30 times more efficient for mesoporous silicon microparticles than for the pSi NPs synthesised using the CO2 laser pyrolysis technique.
PEC cells work by breaking down water molecules into O2 and H2 at the photoanode (n-type) and photocathode (p-type), respectively.4,20 In addition, catalysts can increase the H2 evolution reaction when fabricated with photoelectrodes. There has only been one previous report of PEC water splitting based on the synthesis of n-type pSi NPs using magnesiothermic reduction of silica.25 To our knowledge, p-type pSi NPs have not been investigated for PEC water splitting. It is known that the inclusion of indium phosphide (InP) NCs and an iron sulfur carbonyl (Fe2S2(CO)6) electrocatalyst on a silicon electrode improves PEC efficiency.15,26,27 In this work, we report a facile strategy to fabricate a PEC water-splitting electrode from p-type pSi NPs, obtained from electrochemical etching, coated with InP NCs and Fe2S2(CO)6 catalyst. This is the first example of a pSi NP system sensitised with inorganic nanomaterials as a photocathode for H2 production.
Experimental
Materials
pSi NPs were produced from p-type silicon wafers (Czochralski, Siltronix Ltd.) with resistivity of 0.8–12 mΩ cm, orientation (100). Hydrofluoric acid (HF) (48%) was purchased from Scharlau Chemie (Chem-Supply Pty. Ltd. Australian representation).pSi NPs preparation
pSi NPs are produced via electrochemical anodisation of p-type silicon in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of aqueous (48%) HF and ethanol in an 18 cm2 Teflon cell with a platinum (Pt) counter electrode. A square waveform consisting of a 50 mA cm−2 lower etch current, applied for 7.3 s, was followed by an upper perforate current of 400 mA cm−2, and applied for 0.3 s. This square waveform was continued for 474 repeats (approximately 1 h) after which the HF solution was changed to a 1
1 mixture of aqueous (48%) HF and ethanol in an 18 cm2 Teflon cell with a platinum (Pt) counter electrode. A square waveform consisting of a 50 mA cm−2 lower etch current, applied for 7.3 s, was followed by an upper perforate current of 400 mA cm−2, and applied for 0.3 s. This square waveform was continued for 474 repeats (approximately 1 h) after which the HF solution was changed to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 HF
20 HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol mixture and a 4 mA cm−2 constant current was applied for 250 s to electropolish the pSi film into a free standing membrane. The freestanding pSi film was fractured into pSi NPs by ultra-sonication for 16 h in ethanol purged with N2. The resulting NPs solution was spun at 10
ethanol mixture and a 4 mA cm−2 constant current was applied for 250 s to electropolish the pSi film into a free standing membrane. The freestanding pSi film was fractured into pSi NPs by ultra-sonication for 16 h in ethanol purged with N2. The resulting NPs solution was spun at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g to pellet large particles and the supernatant was filtered through a 200 nm filter membrane and then centrifuged at 22
000 × g to pellet large particles and the supernatant was filtered through a 200 nm filter membrane and then centrifuged at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g. The resulting pellet of pSi NPs was re-dispersed in ethanol.
000 × g. The resulting pellet of pSi NPs was re-dispersed in ethanol.
Surface functionalisation of pSi NPs
The surface functionalisation was achieved by thermally hydrosilylating the pSi NPs in a 1 M toluene solution of allyl mercaptan (AM) at 50 °C for 15 h. The AM hydrosilylated pSi NPs were purified by washing with toluene (three times), vacuum dried and stored in a glove box.28InP NC synthesis
InP NCs were synthesised via a slightly modified procedure from Xu et al.29,30 In a typical synthesis, indium(III) chloride (0.1 mmol), stearic acid (0.1 mmol), zinc undecylenate (0.2 mmol), and hexadecylamine (0.2 mmol) were added to a Schlenk flask. The mixture was initially purged with N2 before 1-octadecene (∼2 ml) was added. The mixture was then vacuum/back filled with N2 before heating to 270 °C. Upon reaching 270 °C, a solution of tris(trimethylsilyl)phosphine (1 ml, 0.1 M) in 1-octadecene was rapidly injected causing the temperature of the solution to drop to 250 °C. The NCs were grown for 10 min at 250 °C forming a deep red solution. The NCs was cooled to room temperature before being washed twice with ethanol (2 × 60 ml) to remove any excess ligands. The NCs were finally re-dispersed in toluene (10 ml).Electrode fabrication
The AM hydrosilylated pSi NPs were sonicated for 1 h in toluene prior to fabrication of the electrodes. To fabricate the electrodes, 100 μl of a 1 mg ml−1 solution of AM hydrosilylated pSi NPs in toluene was added to a gold-coated glass slide via drop casting in a glove box under argon. After the NPs were dried, this deposition process was repeated 5 times. The surface was then gently washed with distilled water to removing any unbound pSi NPs. Following this, 5 layers of InP NCs were added to the surface via drop casting. Finally, the InP NCs sensitised electrode was gently washed with distilled water to remove the unbound InP NCs and a final layer of Fe2S2(CO)6 catalyst (in toluene) was drop-casted on the surface.Surface characterisation
Scanning electron microscopy (SEM) images and energy-dispersive X-ray (EDX) spectra were obtained on a FEI Quanta 450 environmental SEM. Fourier transform infrared spectra (FTIR) spectra were obtained using a Bruker Hyperion 1000 IR microscope operating with a Bruker Vertex 80 IR spectrometer. The IR microscope was equipped with a liquid nitrogen cooled MCT detector. The spectra were collected over 64 scans, with a resolution of 4 cm−1. All spectra were recorded and analysed using OPUS version 7.0 software, in the range of 650–4000 cm−1. Fluorescence microscopy images were recorded with a Nikon Eclipse 50i fluorescence microscope and analysed by NIS Elements software.Photocurrent and gas chromatography measurements
Samples were irradiated using an Abet solar simulator (air mass 1.5–1 sun) and calibrated against a silicon solar cell (New-Spec). Electrochemical measurements were carried out using a PG 310 potentiostat from HEKA Electronics (Germany). Electrolysis was performed using a sealed three-electrode Teflon photoelectrochemical cell consisting of a Pt counter electrode, a Ag|AgCl 3 M KCl reference electrode and the working electrode. The working electrode was illuminated with a light intensity of 100 mW cm−2 under air mass 1.5 conditions with short 12 s dark and 12 s light cycle to measure the photocurrents for 60 s. The potential between the working and reference electrodes was adjusted between 0 and −500 mV in 100 mV steps. The 1 h run photocurrents were measured under a light cycle at a bias voltage of −400 mV with respect to the open circuit potentials (OCPs). The sample gas in the headspace (310 μl) above the electrolyte was sampled after 1 h and analysed using a SRI 310C series gas chromatograph (GC) with a thermal conductivity detector and a column held at 70 °C with N2 as the carrier gas.Results and discussion
pSi NPs
pSi NPs synthesised by CO2 laser pyrolysis20 and mesoporous silicon microparticles synthesised by reducing silicon halogenide24 have previously shown efficient H2 production. These synthesis techniques use silane and silicon halogenide as a starting material to synthesise un-doped pSi NPs and then require a further complex doping process before they can be used as a photocathode material for PEC water splitting. Therefore, we chose to fabricate p-type pSi NPs via straightforward electrochemical anodisation technique. pSi films were fabricated using a square waveform to generate alternating layers of high and low porosity. The highly porous layers are weak and disintegrate rapidly during sonication.11 Fig. S1† shows the top view (A) and cross-sectional (B) SEM images of the pSi film with an average pore size of ∼13 nm and thickness of ∼118 μm. The thickness of the each low porosity layer is ∼240 nm. The pSi membrane was electropolished and removed from the crystalline silicon substrate, which was subjected to sonication for 16 h in ethanol and purged with N2 gas in order to fracture the film into pSi NPs. Fig. 1 shows the SEM image of mesoporous pSi NPs. The inset shows a high-resolution image of the pSi NPs. The average particle size was found to be 161 ± 58 nm with pore sizes of ∼13 nm. | ||
| Fig. 1 SEM image of electrochemically anodised pSi NPs. The inset represents the pSi NPs at a higher magnification. | ||
Fig. 2 shows FTIR of freshly prepared pSi NPs (a) and AM hydrosilylated pSi NPs (b). Spectrum (a) shows the Si–Hx stretching peak at 2100 cm−1 for the bare pSi NPs.31 While spectrum (b) also shows Si–Hx stretching, C–H stretching vibrations at 2900 cm−1 can now be seen which confirms hydrosilylation of the pSi NPs surface.32 The peak at 1100 cm−1 may be attributed to surface oxidation from Si–O–Si groups, after AM hydrosilylation on pSi NPs when compared to the bare pSi NPs. The AM hydrosilylation results in free thiol end groups on the pSi NP surface, providing anchors for attachment to gold surfaces.28,32,33 The inset shows the corresponding fluorescence images of pSi NPs and AM hydrosilylated pSi NPs after reaction with fluorescein-5-maleimide dye (498 nm excitation) in PBS buffer at pH 7. The green fluorescence of AM hydrosilylated pSi NPs after reaction with the dye confirms the availability of thiol groups on the particle surface.28
PEC electrode performance and GC measurements
Fig. S2A and B† show the top-view SEM images of mesoporous AM hydrosilylated pSi NPs and AM hydrosilylated pSi NPs coated with InP NCs and Fe2S2(CO)6 catalyst on gold, respectively. The pores of AM hydrosilylated pSi NPs were covered with InP NCs and Fe2S2(CO)6 catalyst which is evident from Fig. S2B.† Fig. S2C and D† show the cross-sectional SEM images corresponding to Fig. S2A and B.† The thickness of the layer was measured to be 204 ± 33 nm and 212 ± 21 nm for the AM hydrosilylated pSi NPs (Fig. S2C†) and AM hydrosilylated pSi NPs (Fig. S2D†) coated with InP NCs and Fe2S2(CO)6 catalyst on gold, respectively. The presence of C, O, Fe, Si, Au, S, P and In signals in the EDX spectra confirmed the coating of InP NCs and Fe2S2(CO)6 catalyst on the AM hydrosilylated pSi NPs fabricated on gold (Fig. S2E and F†). PEC water splitting was carried out in a sealed three-electrode set up with 0.1 M sulfuric acid (H2SO4) as the electrolyte. H2SO4 was chosen as the electrolyte as it possesses a slightly acidic pH, which helps in improving the electron conduction and contributes more protons for H2 generation.34 A solar simulator provided an artificial sunlight (air mass 1.5 irradiation). Table 1 shows the photocurrent density measurements and corresponding OCPs for the fabricated pSi NPs electrodes. The average photocurrent density measurements for the electrode with AM hydrosilylated pSi NPs alone was ∼−300 nA cm−2, while the hybrid electrode with AM hydrosilylated pSi NPs, InP NCs and Fe2S2(CO)6 catalyst, gave a 5.3 times higher photocurrent density at −1.6 μA cm−2. When a −400 mV bias was applied to the hybrid electrode, an average photocurrent density of −2.1 μA cm−2 was observed. The applied bias photo-to-current efficiency (ABPE) was calculated based on our previous work15 and found to be 0.001%. Our system showed a 1.3 times increase in the photocurrent density for the hybrid pSi NPs electrode with applied bias when compared to the hybrid pSi NPs electrode without applied bias.| Sample | Fabricated electrodes | OCPs | Photocurrent density (μA cm−2) |
|---|---|---|---|
| A | AM-pSi NP on gold | −1.9 ± 1.6 mV | −0.3 ± 0.1 |
| B | AM-pSi NPs + InP NCs + Fe2S2(CO)6 catalyst on gold | −42.5 ± 60.1 mV | −1.6 ± 0.2 |
| C | AM-pSi NPs + InP NCs + Fe2S2(CO)6 catalyst on gold | −112.0 mV (at −400 mV bias) | −2.1 ± 0.2 |
The charge transfer from pSi NPs (1.8 eV band gap) to InP NCs (1.35 eV band gap) and Fe2S2(CO)6 (−0.9 eV band gap) catalyst under illumination assists H2 production. The band gap alignment and charge transport mechanisms are analogous to our previous report using bulk pSi photocathode coated with InP NCs and Fe2S2(CO)6 catalyst.15
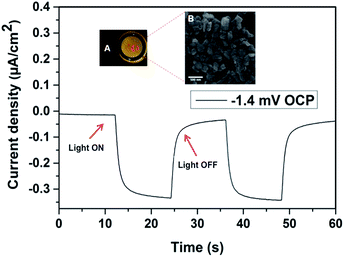
Fig. 3 shows the maximum current density measurements for the AM hydrosilylated pSi NPs on a gold electrode at 0 V and the OCP was measured to be −1.4 mV. The photocurrent density was found to be −300 nA cm−2 and remained stable over several cycles. The inset shows the photographic image (A) and the SEM image (B) of the AM hydrosilylated pSi NPs assembled on a gold electrode.
In Fig. 4 the maximum current density measurements for the hybrid electrode at 0 V to −500 mV in steps of 100 mV are shown. At the bias potential of −500 mV a maximum photocurrent density of −6 μA cm−2 with an OCP of −112 mV was obtained. However, a clear degradation of the photoelectrode was observed under these conditions. Therefore, we chose −400 mV bias potential which showed a stable performance with a maximum photocurrent density of −2.2 μA cm−2. It is also clear that the bias did not affect the photocatalytic performance but just added an electrolytic component.
Fig. 5 shows current density measurements of the hybrid electrode at a bias potential of −400 mV vs. the OCP performed over the course of 1 h when the light was turned on; all other parameters of the photocurrent density measurements were kept constant. The current density trace dropped to −35 μA cm−2 as a result of the increase in the electrolytic activity. The current density slowly decreased with time due to the oxidation of pSi NPs in the slightly acidic electrolyte.34 Unreacted Si–Hx groups remained after AM hydrosilylation on pSi NPs as evident from FTIR spectrum (Fig. 2b). The mechanism of oxidation involves the oxidative hydrolysis of unreacted Si–Hx groups to silicic acid.35,36
 | ||
| Fig. 5 The 1 h current density measurements for hybrid pSi NPs on a gold electrode vs. Ag/AgCl reference electrodes in 0.1 M H2SO4 electrolyte at a bias potential of −400 mV. | ||
To measure the H2 production, 310 μl of gas sample after 1 h was collected from the headspace of the PEC cell and analysed via GC. The peak at 0.533 min (ESI, Fig. S3A†) confirmed the presence of H2.37 Fig. S3B† shows the result of GC analysis of 200 ppm of a H2 standard. From the areas of the sample and standard H2 peaks, the moles of H2 produced were calculated to be approximately 5.84 nmol (see ESI† for calculation). Theoretically, a photocurrent density of −2.2 μA cm−2 should produce 41 nmol of H2 for 1 h of a water splitting reaction. However, the photocurrent density slightly decreased over time, suggesting that the stability of the system needs to be improved. Efforts to improve the stability through surface functionalisation of pSi NPs are currently in progress. The photocurrent density measured for hybrid pSi NPs electrode was less than for a bulk hybrid pSi electrode under identical conditions.15 However, our hybrid pSi NPs electrode achieved a photocurrent density over twice as high as those compared to nanoporous silicon powder coated with tin dioxide.25 We also demonstrated a strategy to fabricate a nanophotocathode using pSi NPs. This example can extend the application of pSi NPs for water splitting to other devices including solar cells and batteries.
Conclusions
We fabricated a PEC water-splitting electrode from p-type pSi NPs coupled with inexpensive inorganic nanomaterials, InP NCs and Fe2S2(CO)6 catalyst. Our system achieved a photocurrent density of approximately −2.2 μA cm−2 and 5.84 nmol of H2 per h was produced. This system provides a significant step forward into developing low cost PEC devices for renewable energy. Efforts focused on the use of various surface passivation techniques, such as thermal hydrocarbonisation and electrografting in order to improve the stability of the pSi NPs photocathode system are underway.Acknowledgements
This work was performed in part at the South Australian node of the Australian National Fabrication Facility (ANFF), a company established under the National Collaborative Research Infrastructure Strategy to provide nano and micro-fabrication facilities for Australia's researchers.Notes and references
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- R. M. Navarro, F. del Valle, J. A. Villoria de la Mano, M. C. Álvarez Galván and J. L. G. Fierro, Adv. Chem. Eng., 2009, 36, 111–143 CAS.
- R. M. Navarro Yerga, M. C. Álvarez Galván, F. del Valle, J. A. Villoria de la Mano and J. L. G. Fierro, ChemSusChem, 2009, 2, 471–485 CrossRef CAS PubMed.
- A. Currao, Chimia, 2007, 61, 815–819 CrossRef CAS.
- T. Hisatomi, T. Minegishi and K. Domen, Bull. Chem. Soc. Jpn., 2012, 85, 647–655 CrossRef CAS.
- M. Matsuoka, M. Kitano, M. Takeuchi, K. Tsujimaru, M. Anpo and J. M. Thomas, Catal. Today, 2007, 122, 51–61 CrossRef CAS PubMed.
- P. V. Kamat and D. Meisel, Curr. Opin. Colloid Interface Sci., 2002, 7, 282–287 CrossRef CAS.
- H. Bahruji, M. Bowker, P. R. Davies, L. S. Al-Mazroai, A. Dickinson, J. Greaves, D. James, L. Millard and F. Pedrono, J. Photochem. Photobiol., A, 2010, 216, 115–118 CrossRef CAS PubMed.
- A. Goetzberger, C. Hebling and H.-W. Schock, Mater. Sci. Eng., R, 2003, 40, 1–46 CrossRef.
- K. Sun, S. Shen, Y. Liang, P. E. Burrows, S. S. Mao and D. Wang, Chem. Rev., 2014, 114, 8662–8719 CrossRef CAS PubMed.
- Z. Qin, J. Joo, L. Gu and M. J. Sailor, Part. Part. Syst. Charact., 2014, 31, 252–256 CrossRef CAS PubMed.
- T. J. Macdonald, Y. J. Mange, M. R. Dewi, H. U. Islam, I. P. Parkin, W. M. Skinner and T. Nann, J. Mater. Chem. A, 2015, 3, 13324–13331 CAS.
- L. Ji, M. D. McDaniel, S. Wang, A. B. Posadas, X. Li, H. Huang, J. C. Lee, A. A. Demkov, A. J. Bard, J. G. Ekerdt and E. T. Yu, Nat. Nanotechnol., 2015, 10, 84–90 CrossRef CAS PubMed.
- B. Kumar, M. Beyler, C. P. Kubiak and S. Ott, Chem.–Eur. J., 2012, 18, 1295–1298 CrossRef CAS PubMed.
- S. Chandrasekaran, T. J. Macdonald, Y. J. Mange, N. H. Voelcker and T. Nann, J. Mater. Chem. A, 2014, 2, 9478–9481 CAS.
- I. Oh, J. Kye and S. Hwang, Bull. Korean Chem. Soc., 2011, 32, 4393 Search PubMed.
- I. Oh, J. Kye and S. Hwang, Nano Lett., 2011, 12, 298–302 CrossRef PubMed.
- K.-Q. Peng, X. Wang, X.-L. Wu and S.-T. Lee, Nano Lett., 2009, 9, 3704–3709 CrossRef CAS PubMed.
- D. P. Tran, T. J. Macdonald, B. Wolfrum, R. Stockmann, T. Nann, A. Offenhäusser and B. Thierry, Appl. Phys. Lett., 2014, 105, 231116 CrossRef PubMed.
- F. Erogbogbo, T. Lin, P. M. Tucciarone, K. M. LaJoie, L. Lai, G. D. Patki, P. N. Prasad and M. T. Swihart, Nano Lett., 2013, 13, 451–456 CrossRef CAS PubMed.
- G. Bernhard, K. Dmitry and S. Olga, Nanotechnology, 2011, 22, 305402 CrossRef PubMed.
- P. Kale, A. C. Gangal, R. Edla and P. Sharma, Int. J. Hydrogen Energy, 2012, 37, 3741–3747 CrossRef CAS PubMed.
- H. Bahruji, M. Bowker and P. R. Davies, Int. J. Hydrogen Energy, 2009, 34, 8504–8510 CrossRef CAS PubMed.
- F. Dai, J. Zai, R. Yi, M. L. Gordin, H. Sohn, S. Chen and D. Wang, Nat. Commun., 2014, 5, 3605 Search PubMed.
- B. H. Meekins, Y.-C. Lin, J. S. Manser, K. Manukyan, A. S. Mukasyan, P. V. Kamat and P. J. McGinn, ACS Appl. Mater. Interfaces, 2013, 5, 2943–2951 CAS.
- T. Nann, S. K. Ibrahim, P.-M. Woi, S. Xu, J. Ziegler and C. J. Pickett, Angew. Chem., Int. Ed., 2010, 49, 1574–1577 CrossRef CAS PubMed.
- T. J. Macdonald and T. Nann, Nanomaterials, 2011, 1, 79–88 CrossRef CAS PubMed.
- S. Chandrasekaran, M. J. Sweetman, K. Kant, W. Skinner, D. Losic, T. Nann and N. H. Voelcker, Chem. Commun., 2014, 50, 10441–10444 RSC.
- S. Xu, J. Ziegler and T. Nann, J. Mater. Chem., 2008, 18, 2653–2656 RSC.
- S. Xu, S. Kumar and T. Nann, J. Am. Chem. Soc., 2006, 128, 1054–1055 CrossRef CAS PubMed.
- J. Nelles, D. Sendor, A. Ebbers, F. Petrat, H. Wiggers, C. Schulz and U. Simon, Colloid Polym. Sci., 2007, 285, 729–736 CAS.
- J. Nelles, D. Sendor, F.-M. Petrat and U. Simon, J. Nanopart. Res., 2010, 12, 1367–1375 CrossRef CAS.
- M. J. Sweetman, C. J. Shearer, J. G. Shapter and N. H. Voelcker, Langmuir, 2011, 27, 9497–9503 CrossRef CAS PubMed.
- S. Chandrasekaran, T. J. Macdonald, A. R. Gerson, T. Nann and N. H. Voelcker, ACS Appl. Mater. Interfaces, 2015, 7, 17381–17387 CAS.
- E. J. Anglin, L. Cheng, W. R. Freeman and M. J. Sailor, Adv. Drug Delivery Rev., 2008, 60, 1266–1277 CrossRef CAS PubMed.
- S. E. Letant, S. Content, T. T. Tan, F. Zenhausern and M. J. Sailor, Sens. Actuators, B, 2000, 69, 193–198 CrossRef CAS.
- K. Zhang, D. Jing, C. Xing and L. Guo, Int. J. Hydrogen Energy, 2007, 32, 4685–4691 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images, EDX spectra, GC analysis and calculation for moles of H2 produced. See DOI: 10.1039/c5ra12559f |
| This journal is © The Royal Society of Chemistry 2015 |