pH and stability of the α-gel phase in glycerol monostearate–water systems using sodium stearoyl lactylate and sodium stearate as the co-emulsifier
Abstract
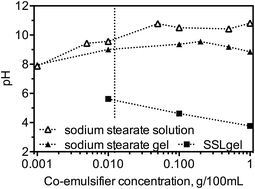
Changing the environmental pH alters the melting profiles of monostearate–water systems and affects the stability of the α-gel phase using sodium stearoyl lactylate (SSL) and sodium stearate (NaS) as co-emulsifiers. NaS in MG-gels may be present both in a micellar phase and in a lamellar phase. Once above the critical micellar concentration of NaS, the NaS solution and diluted MG-gels remained at a stable pH.

 Please wait while we load your content...
Please wait while we load your content...