Synthesis and characterization of 18F-labeled hydrazinocurcumin derivatives for tumor imaging†
Sarah Shinab,
Hyun-Jung Kooa,
Iljung Leea,
Yearn Seong Choe*ab,
Joon Young Choia,
Kyung-Han Leeab and
Byung-Tae Kima
aDepartment of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Ilwon-ro, Kangnam-ku, Seoul 06351, Korea. E-mail: ysnm.choe@samsung.com; Fax: +82-2-3410-2667; Tel: +82-2-3410-2623
bDepartment of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Seoul 06351, Korea
First published on 3rd November 2015
Abstract
Fluorine-substituted hydrazinocurcumin derivative 1 and its dimethyl-substituted form at the C2 and C6 positions (2) were synthesized and their radiolabeled forms, [18F]1 and [18F]2, were evaluated for tumor imaging. In vitro and in vivo metabolism studies showed that the two radioligands were resistant to reductive metabolism, probably due to the presence of a pyrazole ring. In cellular uptake studies, [18F]1 and [18F]2 exhibited comparable uptake by human umbilical vascular endothelial cells and rat C6 glioma cells. Inhibition of radioligand uptake to a similar extent by HC and curcumin suggests that these radioligands may share the same binding sites as those for HC and curcumin. Positron emission tomography imaging of C6 glioma xenografted mice acquired 30 and 60 min after radioligand injection showed that [18F]2 had markedly higher tumor uptake than [18F]1, which was consistent with biodistribution data (3.20 ± 0.35% ID per g vs. 0.98 ± 0.31% ID per g, respectively). However, the two radioligands showed similar levels of tumor-to-background uptake ratio, except for the significantly higher uptake of [18F]1 by the small intestine, indicating its more rapid clearance. The results of this study will guide further structural modifications of these radioligands to enhance tumor-to-background uptake ratios.
Introduction
It is known that 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,4,6-heptatrien-3-one (curcumin), a major ingredient of the curry spice turmeric, has anticancer, antioxidant, and anti-inflammatory activities.1,2 Despite its multiple activities, curcumin has poor bioavailability, which may result from its rapid metabolism in the liver and intestinal walls.3–5 We previously synthesized a curcumin derivative substituted with [18F]fluoropropyl group at one of the 4′-aromatic OH groups for β-amyloid plaque imaging.6 Despite its suitable lipophilicity and excellent in vitro binding affinity for β-amyloid aggregates, [18F]fluoropropylcurcumin showed poor penetration through the blood–brain barrier (BBB), probably due to rapid metabolism of curcumin resulting in formation of its reductive metabolites, conjugated metabolites, and reductive metabolites with conjugation.In addition, curcumin has high reactivity at the central carbon and limited solubility, therefore, a more stable and hydrophilic derivative, 3,5-bis[β-(4-hydroxy-3-methoxyphenyl)-ethenyl]pyrazole (hydrazinocurcumin; HC) has been synthesized and evaluated for diverse biological activities. Initial studies of HC demonstrated a more potent inhibitory activity than curcumin for 5-lipoxygenase (IC50 = 1.0 μM vs. 8.0 μM), which converts arachidonic acid to a host of lipoxygenase products.7 HC was also shown to have antioxidant, cyclooxygenase (COX) inhibitory, and antiinflammatory activities, with even higher activity and COX-2/COX-1 selectivity than curcumin.8 Its activity has been expanded to the field of neurology; HC showed more potent inhibitory activity than curcumin against calcium/calmodulin-dependent protein kinase II, which is known to be associated with various brain functions including learning and memory,9 and also exhibited potent activity against multiple targets associated with pathologic biomarkers of Alzheimer's disease.10 Anticancer activity of HC has been reported, with more potent cytotoxicity (ED50 = 1.0–3.9 μg mL−1) than curcumin against various human cancer cell lines including MCF-7, A549, KB, U87MG, CAKI-1, 1A9, HCT-8, SK-MEl-2, PC-3, HepG2, and LNCaP (clone FGC).11 Antiangiogenesis activity of HC has also been reported, as shown by its inhibitory activity on endothelial cell proliferation and in vivo neovascularization of chorioallantoic membrane.12 Another study showed that HC was more potent than curcumin in inhibiting signal transducer and activator of transcription 3, which in turn resulted in inhibition of cell proliferation, migration and invasion, and induction of cell apoptosis.13 A recent study demonstrated that HC was more effective than curcumin for prevention of diethylnitrosamine-induced hepatocarcinogenesis in rats.14
Recently, radiolabeled curcumin and HC derivatives were developed for β-amyloid plaque imaging and cancer cellular uptake studies. 18F-labeled HC derivative, 2-[3,5-bis(4-hydroxy-3-methoxystyryl)-1H-pyrazol-1-yl]-N-{1-[2-(2-(2-[18F]fluoroethoxy)ethoxy)ethyl-1H-1,2,3-triazol-4-yl]methyl}acetamide had low BBB penetration in wild-type and transgenic APP23 mice, even though it labeled β-amyloid plaques of APP23 mouse brain sections using in vitro autoradiography.15 nat/68Ga-labeled homodimers of curcumin, diacetylcurcumin, and bis(dehydroxy)curcumin were tested for binding affinity to β-amyloid fibrils and uptake by A549 lung cancer cells.16
Unlike curcumin, the in vivo effects of HC and its derivatives have not been extensively studied and there are few reports on their metabolism. We recently found that introduction of methyl groups at both C2 and C6 positions of curcumin significantly diminishes reductive metabolism by creating steric hindrance against reductive metabolizing enzymes.17 In addition, HC as well as curcumin has never been applied to tumor imaging, despite its well-known anticancer activity. In the present study, therefore, we synthesized 4-((E)-2-(5-(4-(2-(2-[18F]fluoroethoxy)ethoxy)-3-methoxystyryl)-1H-pyrazol-3-yl)vinyl)-2-methoxyphenol ([18F]1) and its 2,6-dimethyl derivative ([18F]2), elucidate their metabolism, and evaluated them as tumor imaging agents in a C6 glioma xenograft model using micro positron emission tomography (microPET).
Results
Chemistry
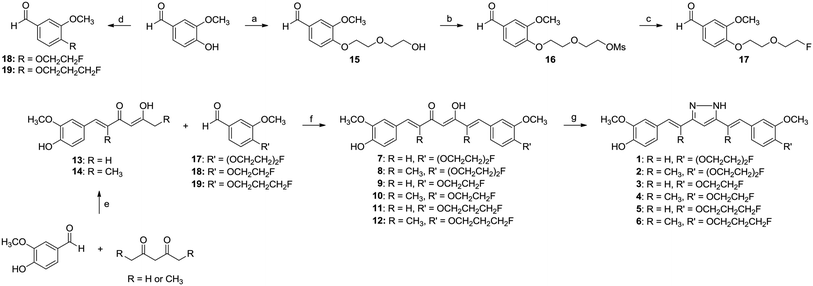
We designed and synthesized six fluorine-substituted HC derivatives for development of 18F-labeled radioligands for tumor imaging (Fig. 1). HC derivatives (1–6) were readily synthesized by hydrazination of curcumin derivatives (7–12) (Scheme 1).7,12 Curcumin derivatives (7–12) were synthesized using a previously reported method with slight modifications;18,19 sequential aldol condensation of 1,3-diketone with one equivalent of vanillin and then with one equivalent of fluoro(ethoxy)alkyl vanillin (17–19) was carried out via coupling of a boron complex of the 1,3-diketone moiety with aldehyde in the presence of amine, followed by acid treatment of the coupling product. Compound 15 and fluoroalkyl vanillin (18 and 19) were synthesized by O-alkylation of vanillin using 2-(2-chloroethoxy)ethanol or fluoroalkyl tosylate in the presence of K2CO3 at 100–110 °C.20 The hydroxyl group of 15 was converted into a methanesulfonyl (Ms) group, which was then reacted with CsF in t-BuOH to give the fluoro-polyethylene glycol (PEG)-substituted vanillin 17.21 The E,E-configurations of the 2,6-dimethyl groups of 2 were confirmed by two-dimensional rotating-frame nuclear Overhauser effect spectroscopy (ROESY) (see 2D ROESY spectra, ESI†).22–24 These experiments showed no cross-peaks between methyl protons at C2 and C6 positions (2.30 ppm) and olefinic protons at C1 and C7 positions (6.98 and 6.99 ppm), indicating that methyl protons do not exist in close proximity (<5 Å) in space with both olefinic protons. However, cross-peaks were observed between methyl protons at C2 and C6 positions and aromatic protons (6.93, 6.92, and 6.90 ppm) and between the methyl protons and a proton at C4 position (6.58 ppm), indicating that methyl protons at C2 and C6 are on the same side as proton at C4. Moreover, the intensities of cross-peaks between the methyl protons and aromatic protons were dependent on the distance between the two protons.23,25 These data demonstrated that the dimethyl groups of 2 are in E,E-configurations. Based on these results, the 2,6-dimethyl HC derivatives synthesized in this study (2, 4, and 6) were assigned to have E,E-configurations.For radiochemical synthesis of [18F]1 and [18F]2, the tosylate precursors 26 and 27 were synthesized (Scheme 2). Compound 20 was synthesized from 13 and pegylated vanillin 15; in this reaction, recrystallization after flash column chromatography was required because compounds 15 and 20 were not separable on flash column chromatography. Subsequent hydrazination of the 1,3-diketone moiety of 20 was readily carried out to yield 22. The bis-methoxymethyl (MOM) protected precursor, 26 was then used for preparation of [18F]1. Precursor 27 for synthesis of [18F]2 was prepared as described for synthesis of 26.
Synthesis of radioligands
[18F]1 and [18F]2 were synthesized by reaction of the corresponding precursors (26 and 27) with n-Bu4N[18F]F at 110 °C for 10 min followed by removal of the protecting groups in 6 N HCl at 120 °C for 10 min (Scheme 2). 18F-labeling of the precursors was readily carried out by protecting the aromatic OH and pyrazole NH groups, unlike the multi-step synthesis of [18F]fluoropropylcurcumin from 4-(3-[18F]fluoropropoxy)-3-methoxybenzaldehyde and 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-1,4-hexadien-3-one.6 Subsequent HPLC purification gave [18F]1 and [18F]2 in overall decay-corrected radiochemical yields of 25–35% and with specific activity of 51.06 GBq μmol−1 and 44.5 GBq μmol−1, respectively.Measurement of partition coefficient
The partition coefficients of [18F]1 and [18F]2 were measured and expressed as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of 2.00 ± 0.01 and 2.11 ± 0.01, respectively. As predicted, lipophilicity of [18F]2 was higher than that of [18F]1, due to the presence of two methyl groups at C2 and C6 positions.
P values of 2.00 ± 0.01 and 2.11 ± 0.01, respectively. As predicted, lipophilicity of [18F]2 was higher than that of [18F]1, due to the presence of two methyl groups at C2 and C6 positions.
Sulforhodamine B (SRB) assay
Cytotoxicity of HC derivatives was measured by carrying out the SRB assay for two cell lines, human umbilical vascular endothelial cells (HUVEC) and rat C6 glioma cells, 72 h after treatment with the compounds (Table 1). All of the compounds showed a similar trend in cytotoxicity for the cell lines studied. HC had superior cytotoxicity to the other compounds including curcumin, which was consistent with results in the literature for bovine aortic endothelial cells.12 The HC derivatives substituted with a mini-PEG moiety (1 and 2) showed more potent cytotoxicity than the other HC derivatives against both HUVECs and C6 glioma cells, and furthermore, 2,6-dimethyl derivative (2) was more potent than the corresponding 2,6-unsubstituted derivative (1). Thus, the two compounds, 1 and 2, were selected for 18F-labeling.| Cell lines | IC50 (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| Curcumin | HC | 1 | 2 | 3 | 4 | 5 | 6 | |
| a Data are mean ± SD from triplicate experiments. | ||||||||
| HUVECs | 7.18 ± 1.65 | 4.34 ± 1.35 | 5.88 ± 1.87 | 4.80 ± 1.31 | 9.77 ± 1.11 | 5.62 ± 1.52 | 10.84 ± 2.53 | 14.70 ± 1.13 |
| C6 glioma cells | 15.95 ± 0.98 | 4.52 ± 1.15 | 17.52 ± 1.59 | 16.35 ± 1.85 | 32.14 ± 2.12 | 22.25 ± 2.15 | 20.99 ± 2.18 | 25.25 ± 3.12 |
Reduction with alcohol dehydrogenase
Reduction of 1 and 2 was performed using alcohol dehydrogenase, an enzyme known to be involved in the reductive metabolism of curcumin.26,27 Compound 1 was eluted at the retention time between 37.9 and 38.9 min (Fig. 2A) and remained intact under complete incubation conditions in the presence of alcohol dehydrogenase and NADPH (Fig. 2B). The peak from 33.0 to 33.8 min was identified as an impurity derived from alcohol dehydrogenase, which was also present in incubation of the enzyme itself (Fig. 2C). Compound 2 was eluted at the retention time between 40.4 and 41.6 min (Fig. 2D) and remained intact under complete incubation conditions (Fig. 2E). Both compounds were also unchanged under conditions omitting NADPH (Fig. 2F). On the other hand, curcumin was mostly converted to tetrahydrocurcumin (Fig. 2G and H), which was confirmed by the same retention time as the authentic tetrahydrocurcumin (Fig. 2I).17In vivo metabolism studies in ICR mice
HPLC chromatograms of the blood and liver samples obtained from mice injected with [18F]1 and [18F]2 showed the same pattern of radiometabolite formation (Fig. 3). Radioligand [18F]2 was metabolically more stable than [18F]1 in blood 5 min after injection, probably due to its higher lipophilicity (Fig. 3a). However, the two radioligands were below detection limit at 30 min except a polar peak at 4.8 min. This rapid metabolism were similar to that of curcumin.28 On the other hand, two radiometabolites were detected in the liver samples of [18F]1 and [18F]2 5 and 30 min after injection (Fig. 3c and d), and there were no radiometabolites detected at 60 min (data not shown). In order to identify whether one of the radiometabolites is a glucuronide conjugate, the 5 min liver samples of [18F]1 were incubated with β-glucuronidase. The results demonstrated that the radiometabolite, m1 (tR = 24.2 min), was hydrolyzed to [18F]1 (tR = 40.1 min) (Fig. 3A-e), suggesting that m1 is [18F]1-glucuronide. Based on the HPLC data of the known metabolites of curcumin, it is likely that m2 is its sulfate conjugate.28Cellular uptake
We carried out the cellular uptake studies of [18F]1 and [18F]2 using two different cell lines, HUVECs and C6 glioma cells. HUVEC uptake of [18F]1 increased in a time-dependent manner from 100% (2.2% ID) at 5 min to 160% (3.5% ID) at 120 min (P < 0.01). Blocking studies demonstrated inhibition of radioligand uptake in the presence of 1, curcumin, and HC by 56%, 64%, and 55%, respectively (P < 0.001) (Fig. 4A and B). Similar results were obtained using C6 cells; uptake increased from 100% (2.8% ID) at 5 min to 163% (4.6% ID) at 120 min (P < 0.01) and radioligand uptake was reduced in the presence of 1, curcumin, and HC by 52%, 52%, and 52%, respectively (P < 0.001) (Fig. 4C and D).HUVEC uptake of [18F]2 increased in a time-dependent manner from 100% (2.6% ID) at 5 min to 165% (4.2% ID) at 120 min (P < 0.001). Blocking studies showed reduction of the radioligand uptake in the presence of 2, curcumin, and HC by 60%, 58%, and 58%, respectively (P < 0.001) (Fig. 4E and F). Similar results were obtained using C6 cells; uptake from 100% (3.2% ID) at 5 min to 153% (4.9% ID) at 120 min (P < 0.01) and reduction of the radioligand uptake in the presence of 2, curcumin, and HC by 61%, 52%, and 49%, respectively (P < 0.001) (Fig. 4G and H).
MicroPET imaging
MicroPET images of C6 glioma xenografted mice were acquired after injection with [18F]1 and [18F]2 dissolved in 0.2% polysorbate 80 in saline, in which polysorbate 80 is a FDA-approved inactive ingredient and is compatible for clinical use. The images revealed accumulation of high levels of radioactivity in the intestines at 30 min post-injection with slow clearance over time. Tumor was not clearly detected in mice injected with [18F]1 (Fig. 5A), whereas it was clearly detected at 30 min after injection of [18F]2 and uptake was retained at 60 min (Fig. 5B). Region of interest (ROI) analysis of tumors in mice injected with [18F]1 revealed 1.10 ± 0.10% ID per g at 30 min and 0.97 ± 0.12% ID per g at 60 min. In contrast, significantly higher ROI values were obtained in tumors of mice injected with [18F]2: 3.30 ± 0.07% ID per g at 30 min (P < 0.01) and 3.90 ± 0.70% ID per g at 60 min (P < 0.001) (Fig. 5C). Tumor to muscle uptake ratios of [18F]1 and [18F]2 were 1.46 and 2.02 at 30 min and 1.28 and 2.28 at 60 min, respectively.Biodistribution
Biodistribution studies carried out immediately after microPET imaging of tumor-bearing mice demonstrated accumulation of high levels of radioactivity in the intestines. Low levels of femur uptake by both [18F]1 and [18F]2 indicated that the radioligands do not undergo in vivo defluorination. Tumor uptake of [18F]1 at 65 min after injection was 0.98 ± 0.31% ID per g, whereas that of [18F]2 was 3.20 ± 0.35% ID per g (Fig. 6). Although tumor uptake of [18F]2 was significantly higher than that of [18F]1 (P < 0.01), tumor-to-background uptake ratios of the two radioligands were within the same range (tumor/muscle and tumor/blood: 2.30 and 0.87 vs. 2.21 and 0.86, respectively). However, it is interesting to note that small intestine uptake was markedly higher for [18F]1 than for [18F]2 (P < 0.05) (Fig. 6).Discussion
A few radiolabeled curcumin and HC derivatives have been developed to date for β-amyloid plaque imaging. Among these radioligands, natGa-labeled homodimers of curcumin derivatives were tested for cancer cellular uptake using their intrinsic fluorescence. However, none of these radioligands have been studied for tumor imaging. Our study as well as other reported studies showed that HC has more potent cytotoxicity than curcumin for various cancer cell lines (Table 1).11 Furthermore, 2,6-dimethylcurcumin was shown to undergo significantly diminished reductive metabolism on olefinic double bonds compared with curcumin, possibly by creating steric hindrance against reductive metabolizing enzymes.17 In this study, therefore, fluorine-substituted HC derivatives (1, 3, and 5) and their 2,6-dimethyl substituted compounds (2, 4, and 6) were synthesized. In compounds 1 and 2, mini-PEG moiety was added between 4′-OH group of HC backbone and 18F to improve their in vivo properties. 18F-PEG has been successfully used to adjust lipophilicity of compounds while retaining their biological activity, as shown in the studies of 18F-PEG-stilbene and styrylpyridines for β-amyloid plaque imaging.29,30As HC is known to have inhibitory activity on endothelial cell proliferation,12 cytotoxicity of HC derivatives was measured using the SRB assay for HUVECs in addition to C6 glioma cells (Table 1). Compounds 1 and 2 were selected for 18F-labeling because of their potent cytotoxicity against the two cells studied. Synthesis of radioligands ([18F]1 and [18F]2) was carried out by labeling bis-MOM protected precursors (26 and 27) with 18F, followed by deprotecting MOM groups. Attempts to prepare [18F]1 by [18F]fluorination of unprotected or 4′-MOM protected tosylate precursor yielded the product in very low yield (<3% based on radio-TLC). Therefore, aromatic OH and pyrazole NH groups of 22 and 23 were selectively protected with MOM groups in the presence of catalytic amounts of Adogen 464®,31,32 and the residual aliphatic OH group was subsequently converted into tosylate ester. The bis-MOM protected precursors were then used for the one-pot synthesis of radioligands in relatively high yields and with high specific activity.
Metabolism of HC derivatives was assessed in vitro and in vivo to determine whether dimethyl substitution on HC could prevent reduction of olefinic double bonds. In vitro reductive metabolism study of 1 and 2 using alcohol dehydrogenase demonstrated that the olefinic double bonds of 1 as well as 2 were not reduced, unlike curcumin.26,27,33 In accord with these results, attempts to synthesize tetrahydro- and hexahydro-forms of compounds 1 and 2 using a previously reported method were not successful, whereas tetrahydro- and hexahydro-curcumin, which are metabolite standards of curcumin, were readily synthesized using the same method.26,33 In vivo metabolism study was performed using the blood and liver samples obtained from mice injected with the radioligands, because a host of endogenous molecules would be also detected by HPLC with UV absorption at 280 nm if non-radioactive compounds 1 and 2 were used instead of radioligands.28 HPLC chromatograms of the liver samples showed that [18F]1 and [18F]2 were mostly biotransformed to two radiometabolites, both of which are most likely to be the conjugation products of the radioligands (Fig. 3). This result was consistent with that obtained from in vitro reduction of 1 and 2 with alcohol dehydrogenase (Fig. 2), suggesting that the pyrazole ring of HC derivatives is responsible for resistance to reductive metabolism because curcumin is known to be a substrate for reductive metabolism.28,34
It was reported that HC has inhibitory activity on endothelial cell proliferation and high antiangiogenesis activity,12,13 and furthermore, compounds 1 and 2 showed cytotoxicity comparable to HC for HUVECs (Table 1). Therefore, we carried out the cellular uptake studies of [18F]1 and [18F]2 using two different cell lines, HUVECs and C6 glioma cells. Blocking studies were carried out using non-radioactive compound (1 or 2), curcumin, and HC. The results demonstrate that cellular uptake of [18F]2 is slightly higher than that of [18F]1, probably because of the presence of two methyl groups in [18F]2, which confer higher lipophilicity than [18F]1. Similar levels of blocking of radioligand uptake by non-radioactive compounds, curcumin, and HC were obtained, suggesting that the radioligands may share the same binding sites as HC and curcumin.
MicroPET images acquired in C6 glioma xenografted mice after injection with radioligands revealed significantly higher radioactivity in the tumor of mice injected with [18F]2 than with [18F]1 (Fig. 5). However, the two radioligands showed similar tumor-to-background uptake ratios, except for the predominantly higher uptake of [18F]1 by the small intestines, indicating its more rapid clearance. Furthermore, the high levels of radioactivity in the intestines of the two radioligands are similar to the known distribution of curcumin in rodents.5 These results suggest that the differences in the in vivo uptake may be attributable to the higher lipophilicity of [18F]2 than [18F]1.
It has been shown that HC has diverse biological activity probably resulting from its interaction with multiple targets.7–14 Our microPET and biodistribution studies demonstrated high retention of radioactivity in tumors of C6 tumor-bearing mice. However, the molecular targets of these radioligands for the tumor uptake have not been identified. Therefore, further investigation may be necessary, not only to enhance the tumor-to-background uptake ratios of these radioligands through structural modifications, but also to elucidate their specific molecular targets.
Conclusion
Of six fluorine-substituted HC derivatives, compounds 1 and 2 were selected for 18F-labeling because of their potent cytotoxicity. Although in vitro cellular uptake of [18F]1 and [18F]2 was comparable, in vivo properties of the radioligands in C6 glioma xenografted mice differed, such that [18F]2 had significantly lower small intestine uptake than [18F]1 but higher uptake in tumors and in other major organs, resulting in similar tumor to muscle and tumor to blood uptake ratios. Furthermore, [18F]1 and [18F]2 were resistant to reductive metabolism in vitro and in vivo, probably because of the presence of a pyrazole ring. To the best of our knowledge, these are the first microPET studies of radiolabeled HC derivatives in tumor xenografted mice.Experimental
Materials and equipment
Chemicals, SRB, equine alcohol dehydrogenase, β-glucuronidase, and polysorbate 80 (10% in water) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 1H NMR spectra were obtained using a Bruker Avance 500 (500 MHz) spectrometer (Rheinstetten, Germany), and chemical shifts (δ) were reported as the ppm downfield of the internal tetramethylsilane. All NMR samples were prepared in CDCl3 or DMSO-d6 at 20 mM, and under these conditions, the NH and OH protons were not detected in all compounds. 13C NMR spectra were obtained on a Bruker Avance 600 (150 MHz) spectrometer. Two-dimensional ROESY experiments were carried out on a Bruker Avance 600 (600 MHz) spectrometer at National Center for Inter-University Research Facilities, Seoul National University (Seoul, Korea). The spectra were acquired using the states-TPPI mode with mixing times of 700 ms and acquisition times of 0.14 s. Electron impact (EI) and fast atom bombardment (FAB) mass spectra were obtained using a JMS-700 Mstation (JEOL Ltd, Tokyo, Japan). Purification and analysis of radioligands were performed using HPLC (Thermo Scientific, Waltham, MA, USA) equipped with a semi-preparative column (YMC-Pack Si, 5 μm, 10 × 250 mm) or an analytical column (YMC-Pack C18, 5 μm, 4.6 × 250 mm). The eluent was monitored simultaneously, using UV (254 nm) and NaI(T1) radioactivity detectors. TLC was performed on Merck F254 silica plates and analyzed on a Bioscan radio-TLC scanner (Washington, D.C., USA).[18F]Fluoride was produced by the 18O(p,n)18F reaction using a GE Healthcare PETtrace cyclotron (Uppsala, Sweden). Radioactivity was measured in a dose calibrator (Biodex Medical Systems, Shirley, NY, USA) and tissue radioactivity was measured in a Wizard2 automatic gamma counter (PerkinElmer, Waltham, MA, USA). MicroPET images were acquired at the Center for Molecular and Cellular Imaging, Samsung Biomedical Research Institute (SBRI, Seoul, Korea) using an Inveon microPET/CT scanner (Siemens Medical Solutions, Malvern, PA, USA).
Synthesis of fluorine-substituted HC derivatives (1, 3, and 5)
Hydrazine hydrate (3 eq.) and catalytic amounts of acetic acid (0.5 eq.) were added to a solution of compound 7 (50 mg, 0.10 mmol), 9 (50 mg, 0.11 mmol), or 11 (50 mg, 0.12 mmol) in ethanol (500 μL), and the reaction solution was heated at 80 °C for 18 h. At the end of the reaction, the reaction mixture was extracted twice with ethyl acetate (30 mL × 2), and the organic layer was washed with a saturated NaHCO3 solution and with brine, and then dried over Na2SO4. The product was purified using flash column chromatography (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 hexane–ethyl acetate) to yield 1 (26.8 mg, 53%), 3 (20.3 mg, 45%), or 5 (37.6 mg, 74%) as a light yellow solid.
2 hexane–ethyl acetate) to yield 1 (26.8 mg, 53%), 3 (20.3 mg, 45%), or 5 (37.6 mg, 74%) as a light yellow solid.
Synthesis of fluorine-substituted 2,6-dimethyl-HC derivatives (2, 4, and 6)
Compounds 2, 4, and 6 were synthesized as described for syntheses of 1, 3, and 5 using 8 (50 mg, 0.10 mmol), 10 (30 mg, 0.06 mmol), or 12 (50 mg, 0.10 mmol) instead of 7, 9, or 11. Flash column chromatography (hexane–ethyl acetate) yielded 2 (34.5 mg, 73%), 4 (26.4 mg, 89%), or 6 (34.6 mg, 58%) as a light yellow solid. Configurations of 2,6-dimethyl groups of 2 were determined by 2D ROESY.Synthesis of fluorine-substituted curcumin derivatives (7, 9, and 11)
B2O3 (0.22 mmol) was added to a solution of 13 (50 mg, 0.21 mmol) in ethyl acetate and the reaction solution was heated at 80 °C for 30 min. The reaction mixture was then reacted with an ethyl acetate solution (700 μL) of 17, 18, or 19 (0.21 mmol) and (n-BuO)3B (0.22 mmol). After stirring at 80 °C for 30 min, piperidine (14.1 μL, 0.14 mmol) was added dropwise to the mixture, which was then stirred at 100 °C for 2 h. The solution was cooled and treated with 0.4 N HCl (700 μL) at 50 °C for 30 min. The reaction mixture was extracted twice with ethyl acetate (30 mL × 2), and the organic layer was washed with water and dried over Na2SO4. The product was purified using flash column chromatography (hexane–ethyl acetate) to give 7 (65.1 mg, 67%), 9 (33.1 mg, 38%), or 11 (54.5 mg, 61%) as a yellow solid.Synthesis of fluorine-substituted 2,6-dimethyl-curcumin derivatives (8, 10, and 12)
Compounds 8, 10, and 12 were synthesized as described for syntheses of 7, 9, and 11 using 14 (100.0 mg, 0.38 mmol) instead of 13. Flash column chromatography (hexane–ethyl acetate) gave 8 (143.2 mg, 78%), 10 (65.8 mg, 39%), or 12 (60.0 mg, 37%) as a light yellow solid.Synthesis of 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-hexa-1,4-dien-3-one and its dimethyl-substituted derivative (13 and 14)
B2O3 (733.8 mg, 10.54 mmol) was added to a solution of 2,4-pentanedione (600.7 mg, 6.00 mmol) in ethyl acetate, and the solution was stirred at 80 °C for 30 min. An ethyl acetate solution (2.7 mL) of vanillin (912.2 mg, 6.00 mmol) and (n-BuO)3B (0.46 mL, 1.70 mmol) was added to the reaction mixture. After stirring at 80 °C for 30 min, n-BuNH2 (0.23 mL, 2.28 mmol) was added dropwise to the mixture, which was then stirred at 80 °C for 1 h. The solution was cooled and treated with 1 N HCl (4.5 mL) at 50 °C for 30 min. The reaction mixture was extracted twice with ethyl acetate (200 mL × 2), and the organic layer was washed with water and dried over Na2SO4. Flash column chromatography followed by recrystallization from ethanol gave 13 (600 mg, 43%) as a yellow solid. Compound 14 was synthesized as described for synthesis of 13 using 3,5-heptanedione. Flash column chromatography (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane–ethyl acetate) yielded 14 (516.9 mg, 33%) as a yellow solid.
1 hexane–ethyl acetate) yielded 14 (516.9 mg, 33%) as a yellow solid.
Synthesis of 4-(2-(2-fluoroethoxy)ethoxy)-3-methoxybenzaldehyde (17)
2-(2-Chloroethoxy)ethanol (1.0 mL, 9.85 mmol) was added to a solution of vanillin (1 g, 6.57 mmol) in DMF (100 mL) in the presence of K2CO3 (1.18 g, 8.54 mmol), and the reaction mixture was stirred at 100 °C for 5 h. At the end of the reaction, the mixture was diluted with water, extracted twice with CH2Cl2 (150 mL × 2), and the combined organic layer was washed with water (50 mL) and with a saturated ammonium chloride solution to remove residual DMF and dried over Na2SO4. Flash column chromatography (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 hexane–ethyl acetate) yielded 15 (986.3 mg, 63%) as a white solid.
5 hexane–ethyl acetate) yielded 15 (986.3 mg, 63%) as a white solid.
Compound 15 (500 mg, 2.08 mmol) was dissolved in CH2Cl2, and Et3N (1.74 mL, 12.48 mmol) was added. The mixture was stirred at room temperature for 1 h, and then MsCl (193.3 mg, 2.49 mmol) was added at 0 °C, and the mixture was stirred at room temperature for 6 h. The reaction was quenched by addition of a saturated ammonium chloride solution (50 mL). The reaction mixture was extracted twice with CH2Cl2 (50 mL × 2), and the combined organic layer was washed with water (50 mL) and dried over Na2SO4. Flash column chromatography (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH2Cl2–methanol) gave 16 (632 mg, 95%) as a white solid.
1 CH2Cl2–methanol) gave 16 (632 mg, 95%) as a white solid.
Compound 16 (500 mg, 1.57 mmol) and cesium fluoride (715 mg, 4.71 mmol) were dissolved in t-BuOH (20 mL), and the reaction mixture was stirred at 100 °C for 19 h. At the end of the reaction, the mixture was diluted with water, extracted twice with ethyl acetate (100 mL × 2), and the combined organic layer was washed with water (100 mL) and dried over Na2SO4. Flash column chromatography (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane–ethyl acetate) yielded 17 (323.8 mg, 85%) as a white solid.
1 hexane–ethyl acetate) yielded 17 (323.8 mg, 85%) as a white solid.
Synthesis of 4-(2-fluoroethoxy)-3-methoxybenzaldehyde (18) and 4-(3-fluoropropoxy)-3-methoxy benzaldehyde (19)
2-Fluoroethyl or 3-fluoropropyl tosylate (4.27 mmol) was added to a solution of vanillin (500 mg, 3.28 mmol) in CH3CN (50 mL) in the presence of K2CO3 (590 mg, 4.27 mmol), and the reaction mixture was stirred at 110 °C for 3 h. At the end of the reaction, the mixture was diluted with water, extracted with ethyl acetate (100 mL), and the combined organic layer was washed with water (100 mL) and dried over Na2SO4. Flash column chromatography (hexane–ethyl acetate) afforded 18 (428.2 mg, 67%) or 19 (595.5 mg, 86%) as a white solid.Synthesis of curcumin derivatives (20 and 21)
B2O3 (128.10 mg, 1.84 mmol) was added to a solution of 13 or 14 (1.84 mmol) in ethyl acetate (2 mL), and stirred at 80 °C for 30 min. An ethyl acetate solution (2 mL) of 15 (300 mg, 1.53 mmol) and (n-BuO)3B (453 μL, 1.68 mmol) was added to the reaction mixture. After stirring at 80 °C for 30 min, piperidine (75 μL, 0.76 mmol) was added to the mixture, which was then stirred at 80 °C for 1 h. The solution was cooled and treated with 0.4 N HCl (2 mL) at 50 °C for 30 min. The reaction mixture was extracted twice with ethyl acetate (100 mL × 2), and the combined organic layer was washed with water (100 mL) and dried over Na2SO4. Flash column chromatography followed by recrystallization from acetone gave 20 (238.5 mg, 34%) or 21 (317.2 mg, 42%) as a yellow solid.Synthesis of HC derivatives (22 and 23)
Hydrazine hydrate (30.95 μL, 0.99 mmol) and acetic acid (18.89 μL, 0.33 mmol) were added to a solution of 20 or 21 (0.33 mmol) in ethanol (3 mL), and the reaction mixture was stirred at 80 °C for 18 h. At the end of the reaction, the reaction mixture was extracted twice with ethyl acetate (30 mL × 2), and the organic layer was washed with a saturated NaHCO3 solution and with brine and then dried over Na2SO4. Flash column chromatography (CH2Cl2–methanol) gave 22 (99.8 mg, 67%) or 23 (119.4 mg, 75%) as a light yellow solid.Synthesis of bis-MOM-protected HC derivatives (24 and 25)
Chloromethyl methyl ether (45.6 μL, 0.60 mmol) was added to a solution of 22 or 23 (0.15 mmol) in CH2Cl2 (877 μL) in the presence of Adogen 464® (17.56 μL) and 2 N NaOH (293 μL), and the reaction mixture was stirred at room temperature for 20 min. At the end of the reaction, the mixture was diluted with water and extracted with CH2Cl2 (20 mL), and the organic layer was then dried over Na2SO4. Flash column chromatography (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 hexane–ethyl acetate) yielded 24 (47.1 mg, 58%) or 25 (45.2 mg, 53%) as a yellow oil.
2 hexane–ethyl acetate) yielded 24 (47.1 mg, 58%) or 25 (45.2 mg, 53%) as a yellow oil.
Synthesis of precursors (26 and 27)
Et3N (12.5 μL, 0.09 mmol) was added to a solution of 24 or 25 (0.06 mmol) in CH2Cl2 (500 μL), followed by addition of p-toluenesulfonyl chloride (TsCl, 13.3 mg, 0.07 mmol). The reaction mixture was stirred at room temperature for 20 h. At the end of reaction, the mixture was diluted with water (20 mL), extracted twice with CH2Cl2 (20 mL × 2), and the organic layer was dried over Na2SO4. Flash column chromatography (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 hexane–ethyl acetate) yielded 26 (31.0 mg, 73%) or 27 (36.6 mg, 83%) as a colorless viscous oil.
2 hexane–ethyl acetate) yielded 26 (31.0 mg, 73%) or 27 (36.6 mg, 83%) as a colorless viscous oil.
Radiochemical synthesis of [18F]1 and [18F]2
[18F]Fluoride (185–1110 MBq) was placed in a Vacutainer containing n-Bu4NHCO3. Three azeotropic distillations were performed using 200 to 300 μL aliquots of CH3CN at 90 °C (oil bath) under a gentle stream of N2. The resulting n-Bu4N[18F]F was dissolved in CH3CN (100 μL) and transferred to a reaction vial containing the precursor 26 or 27 (0.35 μmol), and stirred at 110 °C for 10 min. The reaction mixture was cooled, treated with 6 N HCl (200 μL), and stirred at 120 °C for 10 min. The reaction mixture was cooled, diluted with water (2 mL), and extracted with ethyl acetate (2 mL). The organic layer was washed with water and passed through a 2 cm Na2SO4 plug, and solvent was removed under a stream of N2 at 50 °C (water bath). The crude product was then purified by HPLC using a semi-preparative column eluted with a 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 (or 70
35 (or 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) mixture of hexane and CH2Cl2 (containing 5% 2-propanol) at a flow rate of 3.5 mL min−1. The desired product [18F]1 eluted between 35 and 37 min (or 40 and 42 min for [18F]2) was concentrated under a stream of N2. For cellular uptake and microPET imaging studies, the residue was re-dissolved in polysorbate 80 and diluted with saline to give a final solution of 0.2% polysorbate 80 in saline.
30) mixture of hexane and CH2Cl2 (containing 5% 2-propanol) at a flow rate of 3.5 mL min−1. The desired product [18F]1 eluted between 35 and 37 min (or 40 and 42 min for [18F]2) was concentrated under a stream of N2. For cellular uptake and microPET imaging studies, the residue was re-dissolved in polysorbate 80 and diluted with saline to give a final solution of 0.2% polysorbate 80 in saline.
Specific activity was determined by comparing UV peak areas of the desired radioactive peak and those of different concentrations of unlabeled standard 1 or 2 on HPLC using an analytical column eluted with a 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 mixture of water and acetonitrile each containing 0.1% TFA at a flow rate of 1 mL min−1. Identity of radioligand [18F]1 or [18F]2 was determined by co-injecting the radioligand with the corresponding non-radioactive standard into the HPLC system (see HPLC chromatograms, ESI†).
55 mixture of water and acetonitrile each containing 0.1% TFA at a flow rate of 1 mL min−1. Identity of radioligand [18F]1 or [18F]2 was determined by co-injecting the radioligand with the corresponding non-radioactive standard into the HPLC system (see HPLC chromatograms, ESI†).
Measurement of partition coefficient
Radioligand (0.74 MBq, [18F]1 or [18F]2) was added to a premixed suspension containing 600 μL of octanol and 600 μL of water, vortexed vigorously for 3 min, and then centrifuged. Two layers were separated, and 100 μL aliquots of the octanol and aqueous layers were removed and counted. Samples from the octanol and aqueous layers repartitioned until consistent values were obtained. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P is expressed as the logarithm of the ratio of the counts per minute of octanol versus that of water.
P is expressed as the logarithm of the ratio of the counts per minute of octanol versus that of water.
Cell lines and culture
HUVECs were maintained in endothelial cell growth medium-2 (EGM-2) (Lonza, Walkersville, MD, USA). Rat C6 glioma cells were cultured in Dulbecco's modified Eagle medium (DMEM) (Gibco, Brooklyn, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 1% penicillin/streptomycin (Gibco). Cell lines were maintained at 37 °C in a humidified 5% CO2 incubator.Animals
Animal study was reviewed and approved by the Institutional Animal Care and Use Committee of SBRI. SBRI is an Association for Assessment and Accreditation of Laboratory Animal Care International accredited facility and abide by the Institute of Laboratory Animal Resources Guide.SRB assay
HUVEC and C6 glioma cells were seeded on 96-well plates at a density of 2 × 104 cells per well and incubated for 24 h to allow the cells to attach. The cells were treated with each of the compounds (0, 0.78, 1.56, 3.125, 6.25, 12.5, 25, 50, and 100 μM) for 72 h. All compounds were dissolved in a final concentration of 0.1% DMSO in media. After 72 h, the cells were washed with PBS and fixed with 10% ice-cold trichloroacetic acid at 4 °C for 1 h. Fixed cells were washed five times with distilled water, stained with 0.4% SRB in 1% acetic acid at room temperature for 30 min, and then washed with 1% acetic acid. SRB-bound cells were solubilized with 10 mM Tris buffer (pH 10.5), and the absorbance of the cells was read at a wavelength of 515 nm on an ELISA microplate reader. The IC50 (50% inhibitory concentration) values were calculated using GraphPad prism software.Reduction with alcohol dehydrogenase
Reduction of 1 and 2 with alcohol dehydrogenase was performed as previously reported.26,29 The compound was dissolved in 0.1% DMSO in phosphate buffer (0.01 M, pH 7.4), and the solution was pre-incubated with equine alcohol dehydrogenase (5 units per mL) at 37 °C for 3 min. The reaction was initiated by addition of NADPH (5 mM) at the same temperature. The final volume was 0.5 mL. After 1 h, the incubation mixture was treated with acetate buffer (1 M, pH 4.5) and then extracted with a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of ethyl acetate and 2-propanol. The organic layer was dried, re-dissolved in HPLC solvents, and analyzed by HPLC using an analytical column (YMC C18, 4.6 × 250 mm, 5 μm) and sequential gradients from a 95
1 mixture of ethyl acetate and 2-propanol. The organic layer was dried, re-dissolved in HPLC solvents, and analyzed by HPLC using an analytical column (YMC C18, 4.6 × 250 mm, 5 μm) and sequential gradients from a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mixture to a 55
5 mixture to a 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 mixture of 1% ammonium acetate in water (pH 4.5) and acetonitrile containing 0.05% acetic acid over 30 min, followed by an increase to a 5
45 mixture of 1% ammonium acetate in water (pH 4.5) and acetonitrile containing 0.05% acetic acid over 30 min, followed by an increase to a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 mixture over 20 min at a flow rate of 1 mL min−1. The eluent was monitored using a UV detector at 280 nm.
95 mixture over 20 min at a flow rate of 1 mL min−1. The eluent was monitored using a UV detector at 280 nm.
In vivo metabolism studies in ICR mice
[18F]1 and [18F]2 were injected into ICR mice (male, 32–35 g) via a tail vein. At 5, 30, and 60 min after injection, mice were sacrificed, and samples of blood and liver were obtained, homogenized in 1 mL of ethanol, and centrifuged. The supernatants were passed through filters and then analyzed by HPLC using sequential gradients from a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mixture to a 55
5 mixture to a 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 mixture of 0.1% ammonium acetate in water (pH 4.5) and acetonitrile over 30 min, followed by an increase to a 5
45 mixture of 0.1% ammonium acetate in water (pH 4.5) and acetonitrile over 30 min, followed by an increase to a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 mixture over 20 min at a flow rate of 1 mL min−1. The eluents were monitored using a NaI(Tl) radioactivity detector. In another experiment, the 5 min liver sample was incubated with β-glucuronidase in phosphate buffer (0.01 M, pH 7) at 37 °C for 30 min. The incubation mixture was homogenized in 1 mL of ethanol and centrifuged. The supernatant was analyzed by HPLC using the same conditions as described above.
95 mixture over 20 min at a flow rate of 1 mL min−1. The eluents were monitored using a NaI(Tl) radioactivity detector. In another experiment, the 5 min liver sample was incubated with β-glucuronidase in phosphate buffer (0.01 M, pH 7) at 37 °C for 30 min. The incubation mixture was homogenized in 1 mL of ethanol and centrifuged. The supernatant was analyzed by HPLC using the same conditions as described above.
Cellular uptake
HUVEC and C6 cells were seeded at 5 × 105 cells per well in 12-well plates and incubated for 24 h to allow the cells to attach. [18F]1 or [18F]2 (74 kBq) dissolved in polysorbate 80 (final concentration: 0.1% in medium) was added to each well to give a total volume of 1 mL, and the cells were incubated at 37 °C for 5, 15, 30, 60, and 120 min. At the indicated time points, cells were washed three times with PBS, lysed using 0.1 N NaOH, and the resulting lysate was counted using a gamma counter. For blocking studies, the cells were incubated with [18F]1 or [18F]2 (74 kBq) in the presence of 40 μM compound (1 or 2), curcumin, or HC at 37 °C for 30 min and then treated as described above. All experiments were performed in triplicate.MicroPET imaging
C6 cells (2 × 106) were subcutaneously inoculated into the right hind legs of 5 week-old BALB/c nude mice (male). When tumor size reached 255 ± 21 mm3 at 2.5 weeks after inoculation, [18F]1 or [18F]2 (7.4 MBq/200 μL) in 0.2% polysorbate 80 in saline was injected intravenously into the mice through a tail vein (n = 3). MicroPET images were acquired for 5 min at 30 and 60 min after injection. The images obtained were reconstructed using 3-D ordered subset expectation maximization and then processed using Siemens Inveon Research Workplace 4.1 (IRW 4.1). Regions of interest (ROIs) were drawn over tumors in the right legs and the muscles in contralateral legs, and the average signal level in the ROIs was measured.Biodistribution
At the end of microPET imaging, 65 min after injection of radioligands, the mice were sacrificed. Blood and major tissues were removed, weighed, and their radioactivity counted. Data are expressed as the percent injected dose per gram of tissues (% ID per g).Statistical analysis
The results were analyzed using unpaired, two-tailed Student's t-tests. Differences at the 95% confidence level (P < 0.05) were considered statistically significant.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (grant code: 2011–0030164).Notes and references
- S. Shishodia, K. Misra and B. Aggarwal, in Dietary Modulation of Cell Signaling Pathways, ed. Y. J. Surh, Z. Dong, E. Cadenas and L. Packer, Press, Boca Raton, 2009, vol. 4, pp. 91–99 Search PubMed.
- V. P. Menon and A. R. Sudheer, Adv. Exp. Med. Biol., 2007, 595, 105 CrossRef PubMed.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharm., 2007, 4, 807 CrossRef CAS PubMed.
- G. Shoba, D. Joy, T. Joseph, M. Majeed, R. Rajendran and P. Srinivas, Planta Med., 1998, 64, 353 CrossRef CAS PubMed.
- S. S. Bansal, M. Goel, F. Aqil, M. V. Vadhanam and R. C. Gupta, Cancer Prev. Res., 2011, 4, 1158–1171 CrossRef CAS PubMed.
- E. K. Ryu, Y. S. Choe, K.-H. Lee, Y. Choi and B.-T. Kim, J. Med. Chem., 2006, 49, 6111–6119 CrossRef CAS PubMed.
- D. L. Flynn, T. R. Belliotti, A. M. Boctor, D. T. Connor, C. R. Kostlan, D. E. Nies, D. F. Ortwine, D. J. Schrier and J. C. Sircar, J. Med. Chem., 1991, 34, 518–525 CrossRef CAS PubMed.
- C. Selvam, S. M. Jachak, R. Thilagavathi and A. K. Chakraborti, Bioorg. Med. Chem. Lett., 2005, 15, 1793 CrossRef CAS PubMed.
- M. Mayadevi, D. Sherin, V. Keerthi, K. Rajasekharan and R. Omkumar, Bioorg. Med. Chem., 2012, 20, 6040 CrossRef CAS PubMed.
- R. Narlawar, M. Pickhardt, S. Leuchtenberger, K. Baumann, S. Krause, T. Dyrks, S. Weggen, E. Mandelkow and B. Schmidt, ChemMedChem, 2008, 3, 165 CrossRef CAS PubMed.
- J. Ishida, H. Ohtsu, Y. Tachibana, Y. Nakanishi, K. F. Bastow, M. Nagai, H.-K. Wang, H. Itokawa and K.-H. Lee, Bioorg. Med. Chem., 2002, 10, 3481 CrossRef CAS PubMed.
- J. S. Shim, D. H. Kim, H. J. Jung, J. H. Kim, D. Lim, S.-K. Lee, K.-W. Kim, J. W. Ahn, J.-S. Yoo and J.-R. Rho, Bioorg. Med. Chem., 2002, 10, 2439 CrossRef PubMed.
- X. Wang, Y. Zhang, X. Zhang, W. Tian, W. Feng and T. Chen, Int. J. Oncol., 2012, 40, 1189 CAS.
- P. Elkins, D. Coleman, J. Burgess, M. Gardner, J. Hines, B. Scott, M. Kroenke, J. Larson, M. Lightner and G. Turner, J. Pharm. Biomed. Anal., 2014, 88, 174 CrossRef CAS PubMed.
- J. Rokka, A. Snellman, C. Zona, B. La Ferla, F. Nicotra, M. Salmona, G. Forloni, M. Haaparanta-Solin, J. O. Rinne and O. Solin, Bioorg. Med. Chem., 2014, 22, 2753 CrossRef CAS PubMed.
- M. Asti, E. Ferrari, S. Croci, G. Atti, S. Rubagotti, M. Iori, P. C. Capponi, A. Zerbini, M. Saladini and A. Versari, Inorg. Chem., 2014, 53, 4922 CrossRef CAS PubMed.
- H.-J. Koo, S. Shin, J. Y. Choi, K.-H. Lee, B.-T. Kim and Y. S. Choe, Sci. Rep., 2015, 5, 14205 CrossRef CAS PubMed.
- H. Pabon, Recl. Trav. Chim. Pays-Bas, 1964, 83, 379 CrossRef CAS.
- T. Masuda, H. Matsumura, Y. Oyama, Y. Takeda, A. Jitoe, A. Kida and K. Hidaka, J. Nat. Prod., 1998, 61, 609 CrossRef CAS PubMed.
- I. Lee, J. Yang, J. H. Lee and Y. S. Choe, Bioorg. Med. Chem. Lett., 2011, 21, 5765 CrossRef CAS PubMed.
- D. W. Kim, D.-S. Ahn, Y.-H. Oh, S. Lee, H. S. Kil, S. J. Oh, S. J. Lee, J. S. Kim, J. S. Ryu and D. H. Moon, J. Am. Chem. Soc., 2006, 128, 16394 CrossRef CAS PubMed.
- A. Bax and D. G. Davis, J. Magn. Reson., 1985, 63, 207 CAS.
- E. Ämmälahti, M. Bardet, D. Molko and J. Cadet, J. Magn. Reson., Ser. A, 1996, 122, 230 CrossRef.
- P. Elkins, D. Coleman, J. Burgess, M. Gardner, J. Hines, B. Scott, M. Kroenke, J. Larson, M. Lightner and G. Turner, J. Pharm. Biomed. Anal., 2014, 88, 174 CrossRef CAS PubMed.
- J. Schleucher and S. S. Wijmenga, J. Am. Chem. Soc., 2002, 124, 5881 CrossRef CAS PubMed.
- S. I. Hoehle, E. Pfeiffer, A. M. Sólyom and M. Metzler, J. Agric. Food Chem., 2006, 54, 756 CrossRef CAS PubMed.
- C. R. Ireson, D. J. Jones, S. Orr, M. W. Coughtrie, D. J. Boocock, M. L. Williams, P. B. Farmer, W. P. Steward and A. J. Gescher, Cancer Epidemiol., Biomarkers Prev., 2002, 11, 105 CAS.
- C. Ireson, S. Orr, D. J. Jones, R. Verschoyle, C.-K. Lim, J.-L. Luo, L. Howells, S. Plummer, R. Jukes and M. Williams, Cancer Res., 2001, 61, 1058 CAS.
- W. Zhang, S. Oya, M. P. Kung, C. Hou, D. L. Maier and H. F. Kung, Nucl. Med. Biol., 2005, 32, 799 CrossRef CAS PubMed.
- W. Zhang, M. P. Kung, S. Oya, C. Hou and H. F. Kung, Nucl. Med. Biol., 2007, 34, 89 CrossRef CAS PubMed.
- F. van Heerden, J. van Zyl, G. Rall, E. Brandt and D. Roux, Tetrahedron Lett., 1978, 19, 661 CrossRef.
- T. R. Kelly, C. T. Jagoe and Q. Li, J. Am. Chem. Soc., 1989, 111, 4522 CrossRef CAS.
- S. I. Uehara, I. Yasuda, K. Akiyama, H. Morita, K. Takeya and H. Itokawa, Chem. Pharm. Bull., 1987, 35, 3298 CrossRef CAS.
- M. H. Pan, T. M. Huang and J. K. Lin, Drug Metab. Dispos., 1999, 27, 486 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15380h |
| This journal is © The Royal Society of Chemistry 2015 |