Temperature-responsive hairy particle-supported proline for direct asymmetric aldol reaction in water†
Xinjuan Li*,
Beilei Yang,
Xianbin Jia,
Maoqin Chen and
Zhiguo Hu*
School of Chemistry and Chemical Engineering, The Key Laboratory of Green Chemical Media and Reactions, State Education Ministry of China, Henan Normal University, Xinxiang 453007, P. R. China. E-mail: xinjuanli2009@163.com; zghu@htu.cn
First published on 15th October 2015
Abstract
In this paper, three kinds of hairy particles with different brush structures were prepared and evaluated as chiral catalysts in the direct asymmetric aldol reaction. Compared to other hairy particles, hairy particle (1) grafted with amphiphilic copolymer chains showed a good conversion rate and an outstanding enantioselectivity in pure water. The copolymer brush on the surface of hairy particle (1) contained temperature responsive poly(N-isopropylacrylamide) which underwent a coil-to-globule transition at a lower critical solution temperature (LCST). The brush formed a hydrophobic nanocavity for the organic substrates, and it provided a suitable reaction microenvironment below and above the LCST of PNIPAM. The aldol reaction was promoted by the catalytic system and it showed stable reactivity from 25 °C to 50 °C. The significant advantages of the hairy particle supported catalytic system were that it was more efficient than the corresponding homogeneous polymer supported proline, and it can be recycled five times while maintaining activity and selectivity.
Introduction
One of the most important carbon–carbon bond-forming reactions in synthetic organic chemistry, the asymmetric aldol reaction, has been intensively studied. Since list reported the direct aldol reaction catalyzed by L-proline under mild reaction conditions,1 L-proline has been widely used in asymmetric organic reactions. Its catalytic efficiency in organic solvents such as dimethylformamide, dimethyl sulfoxide and chloroform has been documented. On adding a small amount of water to the polar solvent, the reaction rate can be accelerated. However, when the reaction is carried out with a large amount of water (or in pure water), it always resulted in low yield with low or no enantioselectivity because of the limited affinity between the hydrophobic reactant and hydrophilic catalyst.2–4Asymmetric reactions in aqueous solutions have become an area of fast growing interest recently because water is a safe, cheap, and environmentally friendly medium. Numbers of water-soluble chiral catalysts have been developed and thus contributed to the growth of green chemistry.5–10 In cases, the catalyst was modified so as to be more hydrophobic, such as formed a concentrated organic phase, which resulted in an observed acceleration in the rate of reaction.11,12 Amphiphilic block copolymers have also been extensively studied for their uses in catalytic systems, and they can also provide a protected hydrophobic environment in water.13–20 Amphiphilic copolymers supported catalysts can catalyze asymmetric reaction in water effectively and can be reused. But the catalyst was recycled by precipitating in the organic solvent, the environment would be polluted. During the application of the phase switchable behavior of amphiphilic copolymer, the copolymer will collapse and precipitate for facile recovery.21–23 However, the recovery rate was not very ideal. Although, numerous strategies have been used to immobilize chiral catalysts onto solid-supported materials such as polystyrene (beads), dendrimers, ionic liquids and inorganic particles or crosslinked insoluble polymers,24–27 they usually show relatively poor catalytic efficiency in water-medium. The intrinsic problem in the heterogeneous catalytic systems is the lower degree of exposure to the reactants. It is still a challenging work to synthesize excellent catalytic systems to combine the advantages of both homogeneous catalyst and heterogeneous catalysts in water.
Herein, we synthesized three kinds of hairy particles supported proline by RAFT precipitation polymerization combining with surface-initiated RAFT copolymerization. Hairy particle (1) contained with hydrophobic styrene, N-isopropylacrylamide (NIPAM) and chiral polymer chain; while hairy particle (2) contained with chiral polymer chain and NIPAM, without styrene; and hairy particle (3) contained with chiral polymer chain and styrene, without NIPAM (Fig. 1). Furthermore, the obtained catalysts were used in asymmetric aldol reaction in water, and the catalytic activity, asymmetric selectivity and recyclability were also further studied. Poly(N-isopropylacrylamide) (PNIPAM) is a temperature-responsive polymer that exhibits a sharp phase transition at a lower critical solution temperature (LCST). Use thermo-responsive polymers to load L-proline to prepare “smart” catalysts, so that the catalytic activities can be switched by adjusting the temperature below or above the LCST.22,23
Experimental section
Materials and reagents
Styrene, methacrylic acid (MAA, Aldin, 98%), ethylene glycol dimethacrylate (EGDMA, Alfa Aesar, 98%), and 1,4-dioxane (Jiangtian Chemicals, China) were purified by distillation under vacuum. NIPAM and azobisisobutyronitrile (Chemical Plant of Nankai University, AR) was recrystallized from ethanol. Cumyl dithiobenzoate (CDB)28 and boc-protected O-acrylic hydroxyproline were prepared following the literature procedure,20 and all the other chemicals were used as received.Measurements
1H NMR spectra were recorded on a Bruker AV-400 NMR spectrometer. FT-IR spectra were recorded on a Nicolet NEXUS Fourier transform infrared spectrometer using KBr pellets. Elemental analysis was performed on a Thermo FLASH 1112 elementar. The morphologies and sizes of the samples were characterized by scanning electron microscopy (SEM, JSM-6390LV) and field-emitting scanning electron microscope (FESEM, JEOL-JSM-6700F). The number-average diameter (Dn) was determined by the SEM image. Molecular weights of the polymers were measured with GPC by using PEO as a standard and DMF as a mobile phase, and RI detector was also used. Two shodex LF-404 columns were conditioned at 35 °C and flow rate = 0.3 mL min−1. HPLC analysis was carried out on Agilent TM 1100 HPLC equipment. The static water contact angles of the films that prepared with microspheres were determined as follows: the films of the hairy particles were prepared by casting their suspension solutions in DMF (10 mg mL−1, after ultrasonic dispersion) on clean glass surfaces. After the solvent was allowed to evaporate at ambient temperature and the resulting films were dried at 25 °C under vacuum overnight, a Krüss FM40 Easy Drop contact angle equipment (Germany) was utilized to determine hairy particles' static water contact angles. The dynamic light scattering was conducted on an ALVCGS-3 in order to investigate the particle size of the micelle.Preparation of the hairy particles supported catalyst
The above hairy particles (1.00 g) were dispersed in dry CH2Cl2 (5 mL), and the solution of TFA (5 mL) in CH2Cl2 (5 mL) was dropwise added for 0.5 h at ice bath, then the mixture was stirred for 0.5 h at ambient temperature. After centrifugation, the solid products were thoroughly washed with water and methanol, and then dried at 40 °C under vacuum to obtain final particles.
Elem. anal. of the hairy particle (1): C, 59.74; H, 7.29; N, 1.45; S, 3.08% (the proline loading was 0.38 mmol g−1).
Elem. anal. of the hairy particle (2): C, 61.69; H, 7.45; N, 1.36; S, 2.32% (the proline loading was 0.43 mmol g−1).
Elem. anal. of the hairy particle (3): C, 60.73; H, 7.42; N, 0.46; S, 2.68% (the proline loading was 0.33 mmol g−1).
The three free copolymers were also deprotected according to the above ways. After reaction, the free polymer solution was concentrated and purified by precipitation in diethyl ether, filtered and dried at 30 °C under vacuum for 48 h, providing the homogeneous polymer supported proline.
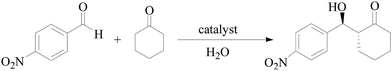
General procedure for the asymmetric aldol reaction
Different substituted benzaldehyde (0.25 mmol) and cyclohexanone (104 μL, 1.0 mmol) were dissolved in solvent (1 mL), and then the catalyst was added. After the reaction, the reaction mixture was isolated by centrifugation, and the hairy particles were washed with ethyl acetate and dried in a vacuum to use again. The aqueous layer was extracted into ethyl acetate, and the organic layers were combined and dried over MgSO4. The solvent was removed under vacuum, and the crude residue was purified by flash column chromatography on silica gel and yielded the pure aldol product. The diastereomeric ratio (dr) was determined by 1H NMR spectroscopy, and the enantiomeric excess (ee) was determined by chiral HPLC.Results and discussion
Preparation of the hairy particles supported catalyst
The poly(MAA-co-EGDMA) microspheres with surface-immobilized dithioester groups were prepared via RAFT precipitation polymerization (RAFTPP) following the ref. 28 and 29. Furthermore, three kinds of hairy particles were synthesized by the modification of the preformed polymer microspheres via surface-initiated reversible addition-fragmentation chain transfer (RAFT) copolymerization. Hairy particle (1) was synthesized by copolymerization with N-boc protected chiral monomer, NIPAM and styrene; hairy particle (2) with chiral monomer, NIPAM but no styrene; hairy particle (3) with chiral monomer, styrene but no NIPAM (Fig. 1). The synthesized method was the same as follows: poly(MAA-co-EGDMA) microspheres as chain transfer agents (CTA), AIBN as the initiator, and 1,4-dioxane as the solvent. A certain amount of CDB was added into the reaction system as the sacrificial chain transfer agent in order to increase the control over the polymerization.30 The weight increases of 16.7 wt%, 14.6 wt% 14.3 wt% were observed for microspheres.The synthesis of statistical copolymers will lead to different products depending on whether the reaction takes place in solution or from the surface of a polymeric particle, due to the different kinetics of copolymerization. So, we investigated the structure of the polymer grafted on the particles and the free polymer generated in the surface-initiated RAFT polymerization system (due to the addition of sacrificial chain transfer agent), and the results were listed in Table 1. The free copolymer ratio for hairy particle (1) was 0.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.21
0.21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15, and the grafted polymer ratio was 0.56
0.15, and the grafted polymer ratio was 0.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.27
0.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.17. The polymer mole ratio on the particle was different from the free polymer. However, the difference was not very obvious. The other hairy particles also got the same results. So the free polymer can be utilized to represent those of the grafted polymer brushes.31,32
0.17. The polymer mole ratio on the particle was different from the free polymer. However, the difference was not very obvious. The other hairy particles also got the same results. So the free polymer can be utilized to represent those of the grafted polymer brushes.31,32
| Free copolymer ratio | Mn, GPC (free polymer) | PDI, GPC (free polymer) | Grafting copolymer ratiod | Grafting density (chain per nm2) | |
|---|---|---|---|---|---|
a Molar mass value of [NIPAM]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [St] [St]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [N-boc protecting chiral polymer] determined by 1H NMR.b Molar mass value of [NIPAM] [N-boc protecting chiral polymer] determined by 1H NMR.b Molar mass value of [NIPAM]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [N-boc protecting chiral polymer] determined by 1H NMR.c [St] [N-boc protecting chiral polymer] determined by 1H NMR.c [St]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [N-boc protecting chiral polymer] determined by 1H NMR.d Molar mass value of grafting copolymer calculated from the relation [NIPAM]grafted = [NIPAM]0 − [NIPAM]free polymer, [St]grafted and [N-boc protecting chiral polymer]grafted were determined by the same way. [N-boc protecting chiral polymer] determined by 1H NMR.d Molar mass value of grafting copolymer calculated from the relation [NIPAM]grafted = [NIPAM]0 − [NIPAM]free polymer, [St]grafted and [N-boc protecting chiral polymer]grafted were determined by the same way. |
|||||
| Hairy particle 1 | 0.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.21 0.21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15a 0.15a |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.43 | 0.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.27 0.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.17a 0.17a |
0.96 |
| Hairy particle 2 | 0.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.33b 0.33b |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.31 | 0.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.28b 0.28b |
0.83 |
| Hairy particle 3 | 0.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.47c 0.47c |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.52 | 0.59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.41c 0.41c |
0.82 |
The free N-boc protected polymers were characterized with 1H-NMR (Fig. 3a, c and e), and the percentage content of the copolymer 1 was determined by comparing distinct polymer signals (δ 1.2–1.5 ppm for boc-protected O-acrylic hydroxyproline Hd, δ 6.2–7.3 ppm for styrene Hb, and δ 3.86 ppm for NIPAM Ha). The ratio of different composition (NIPAM, styrene and N-boc-L-proline) was 0.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.21
0.21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15, with an average molecular weight of 43
0.15, with an average molecular weight of 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, PDI = 1.43 (GPC analysis). The free copolymer 2 without styrene contained 33% of N-boc chiral monomer under a good polymerization control (Mn(GPC) = 50
100, PDI = 1.43 (GPC analysis). The free copolymer 2 without styrene contained 33% of N-boc chiral monomer under a good polymerization control (Mn(GPC) = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, Mw/Mn = 1.19). The free copolymer 3 without NIPAM, contained 47% of N-boc chiral monomer with an average molecular weight of 31
100, Mw/Mn = 1.19). The free copolymer 3 without NIPAM, contained 47% of N-boc chiral monomer with an average molecular weight of 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, PDI = 1.52.
200, PDI = 1.52.
The N-boc protecting groups for the amine can be readily deprotected under acidic conditions.33 Thus, the grafted chiral polymer brushes were deprotected in dry CH2Cl2 in the presence of trifluoroacetic acid (TFA) to afford the final hairy particle. Successful deprotection was confirmed by comparing the 1H NMR signals of the free polymer given in Fig. 3. The tBu signals (at 1.2–1.5 ppm) disappeared following deprotection, and it turned out that the deprotection of hairy particle was successful. The morphology and particle size of the final hairy particles were characterized with SEM (Fig. 2), and the number-average diameters (Dn) of the hairy particles were determined to be 2.78, 2.83 and 2.76 μm, an increases of 320 nm, 370 nm and 318 nm in Dn values were obtained for the grafted polymer microspheres, from which the layer's thickness of 160 nm, 185 nm and 158 nm (i.e., ΔDn/2) could be derived from the grafted polymer brushes. The FESEM image (Fig. 2e, f and g) showed particles coated with a thin polymer shell, which further prove that the polymer chains successfully grafted on the surface of particles. FT-IR was also employed to characterize the grafted polymer microspheres (Fig. 4). It can be seen clearly that in addition to the peaks corresponding to the ungrafted one, some characteristic peaks such as the amide I band (1529 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), amide II band (1627 cm−1, C–N stretching) were also observed, to further verify the successful grafting of chiral polymer brushes. The N contents of hairy particles for elemental analysis were 1.45 wt%, 1.36 wt% and 0.46 wt%, and according to the content of proline in copolymer, the corresponding catalyst loadings were 0.38 mmol, 0.43 mmol and 0.33 mmol catalyst per g particles.
O stretching), amide II band (1627 cm−1, C–N stretching) were also observed, to further verify the successful grafting of chiral polymer brushes. The N contents of hairy particles for elemental analysis were 1.45 wt%, 1.36 wt% and 0.46 wt%, and according to the content of proline in copolymer, the corresponding catalyst loadings were 0.38 mmol, 0.43 mmol and 0.33 mmol catalyst per g particles.
The average surface grafting density can be calculated according to the ref. 27, an average surface grafting density of about 0.96, 0.83 and 0.82 chains per nm2 can be derived for the grafted polymer microspheres (in Table 1). The results indicated the grafted chiral copolymer is in a densely brushy state, and the difference of the grafting density was small.
The hydrophobic and hydrophilic performances of hairy particles were investigated from static water contact angles. It was anticipated that the hairy particle (2) grafted with hydrophilic polymer brushes should show much reduced static water contact angles, while hairy particle (3) grafted with hydrophobic polymer brushes should show much higher static water contact angles. The experimental results indeed supported this hypothesis (Fig. 5). Hairy particle (1) with amphiphilic copolymer chain has the contact angles at 126.1°, which contains some hydrophobic performance. The result provided strong evidence for the presence of different polymer shells on the modified polymer microspheres.
 | ||
| Fig. 5 Profiles of a water drop on the films of the grafted polymer microspheres (1) hairy particle (1), (2) hairy particle (2), (3) hairy particle (3). | ||
The hairy particles supported organocatalyst for the asymmetric aldol reaction
The catalytic activity of the synthesized hairy particles supported L-proline was tested by using a representative aldol reaction between cyclohexanone and 4-nitrobenzaldehyde. The reactions were first carried out with 10 mol% catalyst loading at different temperatures in pure water for 72 h. PNIPAM has been widely studied mainly because of the sharpness of its phase transition and the closeness of its LCST about 32 °C.26,27 We chose this reaction temperature range to study the different possible states of the catalyst system (Table 2): (i) the occurrence of micelles at 0 and 25 °C (i.e., below the LCST), (ii) a little above the LCST at 35 °C, and (iii) far above the LCST at 50 °C. The hairy particle (1) as catalyst has good catalytic activity and stereoselectivity with 84% conversion, 91/9 dr (anti/syn) and >99% ee at 0 °C. Increasing the reaction temperature from 0 to 35 °C, the conversions increased obviously, but the diastereoselectivity and ee values changed little. When further increasing temperature from 35 °C to 50 °C, the conversion almost had no change, but the selectivity decreased obviously. The conversion was the highest (97%) at 35 °C, and with a good selectivity (anti/syn 89/11, ee 97%). Hairy particle (3) with hydrophobic part showed weak catalytic activity at lower temperature. Increasing reaction temperature from 0 to 50 °C, the conversions increased sharply (from 10% to 80%), and the reason can be attributed to the increasing solubility at higher temperature. According to the ref. 34, L-Pro attached to a folded PEG based amphiphilic polymer did show good activity in water, but not very ideal selectivity (eeanti = 72%). This ee value was attributed to a too hydrophilic environment in the vicinity of the catalyst. In the absence of a hydrophobic pocket by using hairy particle (2) at 10 mol% catalyst loading, the aldol reaction in water reached <32% yield at different temperatures, the regioselectivity was also moderate (ee <87%), supporting the effect of the hydrophobic pocket for efficient catalysis.35| Catalysts | Temperature (°C) | % Conversiona | syn/antia | % eeb (anti) |
|---|---|---|---|---|
| a Determined by 1H NMR spectroscopic analysis of the crude product.b Determined by HPLC using a chiral column. | ||||
| Hairy particles (1) | 0 | 84 | 9/91 | >99 |
| Hairy particles (1) | 25 | 90 | 10/90 | 95 |
| Hairy particles (1) | 35 | 97 | 11/89 | 97 |
| Hairy particles (1) | 50 | 97 | 22/78 | 93 |
| Hairy particles (2) | 0 | 5 | 20/80 | 87 |
| Hairy particles (2) | 25 | 32 | 27/73 | 53 |
| Hairy particles (2) | 35 | 16 | 20/80 | 77 |
| Hairy particles (2) | 50 | 17 | 28/72 | 42 |
| Hairy particles (3) | 0 | 10 | 21/79 | 88 |
| Hairy particles (3) | 25 | 67 | 12/88 | 95 |
By contrast, hairy particle (1) has the excellent catalytic activity and stereoselectivity, and our design is successful in loading L-proline at a well defined interface between the hydrophobic and hydrophilic portions of the grafted polymers. Comparison of above catalysis results with recent literature examples, although L-Pro-based block copolymers or nanogels reported by O'Reilly and coworkers show good catalytic results at low catalyst loading (1 mol%), our catalytic system needs more catalyst loading (10 mol%). However, our catalytic system could be easily separated after reaction, to be reused for the subsequent catalysis cycle.
In order to further investigate the activity and selectivity of the hairy particle (1), we compared the catalytic performance of the hairy particle (1) supported system with the corresponding free polymer supported proline system. Fig. 6 shows the kinetics of the reaction catalyzed by the hairy particle (1) and the corresponding free copolymer. Although the differences in stereoselectivity and final conversion after 72 hours are not significant, it can be observed that hairy particle (1) is more effective than free polymer supported system at 10 mol% loading at different temperature from 25 to 50 °C. These results have shown the advantage of the hairy particles supported system. From the reaction kinetics, we also find the reaction rate increased with increasing reaction temperature. It is well known that PNIPAM is a typical thermoresponsive polymer, which undergoes a coil-to-globule transition at the lower critical solution temperature (LCST) at about 32 °C. The smart polymer of PNIPAM is always chosen as the copolymer chains for two reasons. First, the PNIPAM chains become hydrophobic and collapse as core, which can provide a nano environment for hydrophobic guest molecules in water just by increasing the temperature above its phase transition temperature.36 Second, PNIPAM can be recycled due to the reversible phase transition. In this paper, the copolymer chains grafting on hairy particle (1) contain hydrophobic styrene and hydrophilic NIPAM, which form micelles below the phase transition temperature and provide a nanoreactor on the surface of particles. When the temperature increases above the phase transition temperature, the PNIPAM chains collapse and provide a hydrophobic nano cavity for the organic substrates. So the aldol reaction can be aroused above and below the transition temperature of PNIPAM.
 | ||
| Fig. 6 Kinetics for aldol reaction catalyzed by hairy particle (1) and the corresponding free polymer at 10 mol% catalyst loading at different temperatures. | ||
In order to ascertain the contribution of the grafted copolymer on the catalytic property of hairy particle (1), the thermo-responsive conformation changes of free polymer (1) were monitored by TEM (Fig. S1†) and DLS (Fig. 7). The TEM observation showed that, at low temperature (25 °C), micelles containing a hydrophobic backbone core and a PNIPAM corona were formed in the solution of brush copolymers. Micelles in random brush copolymer solution showed irregular shape, and the diameter was approximate 35–45 nm. DLS was carried out with a 3 mg mL−1 free copolymer (1) solution from 25 to 50 °C. As shown in Fig. 7, the insets concerned the DLS spectra of the intensity distribution, and the copolymer showed a hydrodynamic diameter (dH) (116.98 nm) in water at 25 °C. PNIPAM was soluble in water at low temperature, thus, the peak is mostly at 25 °C, which probably indicates the formation of the micelles. However, above the transition temperature, with the temperature increasing persistently, the dH went through a decrease with a lower dH (63.7 nm) at 35 °C, which may result from the collapse of the aggregates by removing more water molecules and forming more compact and regular structures. When the temperature increased to 50 °C, the size of micelles increased (dH = 79.98 nm) and the peak changed broadly. There is almost no obvious shrinkage in the corona of micelles when the temperature is far above LCST. It is certain that the strong repulsive interaction among the denser PNIPAM side chains within micelle leads to the above phenomenon.37
We also investigated the reaction between cyclohexanone and several substituted benzaldehydes with hairy particle (1) as catalyst. In contrast, o-NO2C6H4CHO showed a lower conversion and ee value (entry 2 in Table 3), the reaction gave the best conversion when p-NO2C6H4CHO was used as the reaction substrate, and m-NO2C6H4CHO showed the best stereoselectivity.
| Entry | Aldehyde | Conversion (%) | syn/anti | ee (%) |
|---|---|---|---|---|
| 1 | p-NO2C6H4CHO | 97 | 11/89 | 97 |
| 2 | o-NO2C6H4CHO | 57 | 50/50 | 75(anti), 58(syn) |
| 3 | m-NO2C6H4CHO | 68 | 1/99 | 98% |
Recovery and recycling of the catalytic system were next investigated. The potential recycling of the hairy particle (1) supported catalytic system was studied by using the same aldol reaction between 4-nitrobenzaldehyde and cyclohexanone. This adjustment was done to keep the aldol reaction at 10 mol% catalyst loading throughout different cycles at 25 °C (Table S1†) and 35 °C (Fig. 8). The catalyst was successfully used in multiple cycles without losing significant activity or selectivity, and ee value > 91% in each cycle, and only slightly decreases in conversion and enantioselectivity were observed. The recovery rate of the hairy particles was very high (>97%). FTIR also proved that the structure of the chiral polymer chain was not changed after 5 cycles (Fig. 4c).
 | ||
| Fig. 8 Recycle data for the aldol reaction using the hairy particles (1) supported catalyst at 35 °C for 72 h. | ||
Conclusions
This paper proves that RAFT precipitation polymerization combining with surface-initiated RAFT polymerization is an efficient approach to obtain hairy particles supported proline system. The catalysts were well-defined copolymers of styrene, NIPAM and L-proline functionalized chiral monomer, which can catalyst the direct aldol reaction in water efficiently. PNIPAM is a temperature-responsive polymer, which undergoes a coil-to-globule transition at a lower critical solution temperature (LCST) and provides a different reaction interface. The experimental results also proved that the aldol reaction can be accelerated both above and below LCST (32 °C) of poly(N-isopropylacrylamide) and showed a good catalytic activity as well as an asymmetric selectivity from 25 °C to 50 °C. The reaction catalyzed by the hairy particles (1) supported system was more efficient than the homogeneous polymer supported proline. The hairy particles grafting with amphiphilic copolymer chains and temperature responsive polymer chains can provide a new and practical way for chiral catalyst load in pure water.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21204019), the Post-doctoral Foundation of China (No. 2012M521398), the Post-doctoral Foundation of Henan Province and the International Cooperation Project of Henan Province (No. 134300510055).Notes and references
- B. List, R. A. Lerner and C. F. Barbas III, J. Am. Chem. Soc., 2000, 122, 2395–2396 CrossRef CAS.
- R. Millet, A. M. Träff, M. L. Petrus and J.-E. Bäckvall, J. Am. Chem. Soc., 2010, 132, 15182–15184 CrossRef CAS PubMed.
- L. Albrecht, H. Jiang, G. Dickmeiss, B. Gschwend, S. G. Hansen and K. A. Jørgensen, J. Am. Chem. Soc., 2010, 132, 9188–9196 CrossRef CAS PubMed.
- Y. Hayashi, H. Gotoh, M. Honma, K. Sankar, I. Kumar, H. Ishikawa, K. Konno, H. Yui, S. Tsuzuki and T. Uchimaru, J. Am. Chem. Soc., 2011, 133, 20175–20185 CrossRef CAS PubMed.
- Y. Kong, R. Tan, L. L. Zhao and D. D. Yin, Green Chem., 2013, 15, 2422–2433 RSC.
- A. Psarra, C. G. Kokotos and P. Moutevelis-Minakakis, Tetrahedron, 2014, 70, 608–615 CrossRef CAS PubMed.
- K. Armacost and O. Acevedo, J. Am. Chem. Soc., 2014, 136, 147–156 CrossRef CAS PubMed.
- J. Mlynarski and S. Bas, J. Am. Chem. Soc., 2014, 43, 577–587 CAS.
- N. Mase, Y. Nakai, N. Ohara, H. Yoda, K. Takabe, F. Tanaka and C. F. Barbas III, J. Am. Chem. Soc., 2006, 128, 734–735 CrossRef CAS PubMed.
- S. Aratake, T. Itoh, T. Okano, N. Nagae, T. Sumiya, M. Shoji and Y. Hayashi, Chem.–Eur. J., 2007, 13, 10246–10256 CrossRef CAS PubMed.
- M. Amedjkouh, Tetrahedron: Asymmetry, 2007, 18, 390–395 CrossRef CAS PubMed.
- D. E. Siyutkin, A. S. Kucherenko and S. G. Zlotin, Tetrahedron, 2009, 65, 1366–1372 CrossRef CAS PubMed.
- B. M. Rossbach, K. Leopold and R. Weberskirch, Angew. Chem., Int. Ed., 2006, 45, 1309–1312 CrossRef CAS PubMed.
- M. Bortenschlager, N. Schoellhorn, A. Wittmann and R. Weberskirch, Chem.–Eur. J, 2007, 13, 520–528 CrossRef CAS PubMed.
- I. M. Okhapkin, E. E. Makheava and A. R. Khokhlov, Adv. Polym. Sci., 2006, 195, 177–210 CrossRef CAS.
- K. T. Kim, J. J. L. M. Cornelissen, R. J. M. Nolte and J. C. M. van Hest, Adv. Mater., 2009, 21, 2787–2791 CrossRef CAS PubMed.
- T. H. Ku, M. P. Cheng, M. P. Thompson, R. S. Sinkovits, N. H. Olson, T. S. Baker and N. C. Gianneschi, J. Am. Chem. Soc., 2011, 133, 8392–8395 CrossRef CAS PubMed.
- A. D. Ievins, X. F. Wang, A. O. Moughton, J. Skey and R. K. O'Reilly, Macromolecules, 2008, 41, 2998–3006 CrossRef CAS.
- A. C. Evans, A. Lu, C. Ondeck, D. A. Longbottom and R. K. O'Reilly, Macromolecules, 2010, 43, 6374–6380 CrossRef CAS.
- P. Cotanda, A. Lu, J. P. Patterson, N. Petzetakis and R. K. O'Reilly, Macromolecules, 2012, 45, 2377–2384 CrossRef CAS.
- M. O. Simon and C. J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC.
- X. W. Wu, Y. F. Hu, X. H. Wang and L. Chen, Catal. Commun., 2015, 58, 164–168 CrossRef CAS PubMed.
- X. W. Wu, X. F. Chen, H. Y. Guan, X. H. Wang and L. Chen, Catal. Commun., 2014, 51, 29–32 CrossRef CAS PubMed.
- C. S. Gill, W. Long and C. W. Jones, Catal. Lett., 2009, 131, 425–431 CrossRef CAS.
- W. Long and C. W. Jones, ACS Catal., 2011, 1, 674–681 CrossRef CAS.
- B. Zhao, X. M. Jiang, D. J. Li, X. G. Jiang, T. G. O'Lenick, B. Li and C. Y. Li, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3438–3446 CrossRef CAS PubMed.
- X. J. Li, M. Q. Chen, B. L. Yang, S. L. Zhang, X. B. Jia and Z. G. Hu, RSC Adv., 2014, 4, 43278–43285 RSC.
- G. Q. Pan, Y. Zhang, X. Z. Guo, C. X. Li and H. Q. Zhang, Bioelectronics, 2010, 26, 976–982 CrossRef CAS PubMed.
- B. Y. Zu, G. Q. Pan, X. Z. Guo, Y. Zhang and H. Q. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3257–3270 CrossRef CAS PubMed.
- Z. S. An, Q. H. Shi, W. Tang, C. K. Tsung, C. J. Hawker and G. D. Stucky, J. Am. Chem. Soc., 2007, 129, 14493–14499 CrossRef CAS PubMed.
- K. L. Robinson, M. A. Khan, M. V. de Paz Banez, X. S. Wang and S. P. Armes, Macromolecules, 2001, 34, 3155–3158 CrossRef CAS.
- E. Huerta, B. van Genabeek, P. J. M. Stals, E. W. Meijer and A. R. A. Palmans, Macromol. Rapid Commun., 2014, 35, 1320–1325 CrossRef CAS PubMed.
- C. Y. Hong, Y. Z. You and C. Y. Pan, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2419–2427 CrossRef CAS PubMed.
- N. N. Laura, M. B. B. Neumann, M. A. L. Christianus, K. V. Ilja, P. M. L. René, R. A. P. Anja and E. W. Meijer, Org. Biomol. Chem., 2015, 13, 7711–7719 Search PubMed.
- T. P. Le, G. Moad, E. Rizzardo and S. H. Thang, PCT Int. Appl. WO 98/01478, 1998.
- A. Lu, T. P. Smart, T. H. Epps III, D. A. Longbottom and R. K. O'Reilly, Macromolecules, 2011, 44, 7233–7241 CrossRef CAS PubMed.
- J. Škvarla, J. Zednik, M. Šlouf, S. Pispas and M. Št.spasl, Eur. Polym. J., 2014, 61, 124–132 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16393e |
| This journal is © The Royal Society of Chemistry 2015 |