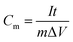One-pot synthesis of 3D flower-like heterostructured SnS2/MoS2 for enhanced supercapacitor behavior
Lina Wangab,
Ying Maab,
Min Yanga and
Yanxing Qi*a
aState Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, PR China. E-mail: wanglina1106@126.com; qiyx@lzb.ac.cn; Fax: +86 931 4968190; Tel: +86 931 4968190
bUniversity of Chinese Academy of Sciences, Beijing 100039, PR China
First published on 15th October 2015
Abstract
Novel three-dimensional flower-like heterostructured SnS2/MoS2 was produced via a one-step hydrothermal method. The full potential of the heterostructured SnS2/MoS2 material could be realized because of a strong synergetic effect, which was not only able to effectively weaken the agglomeration and restacking problems during the electrochemical reaction, but also able to ensure the high-rate and long-life. We found that SnS2/MoS2 had better electrochemical performance compared to MoS2, due to the rapid electronic transport and volume change buffering of the formation of the SnS2/MoS2 heterostructure. The electrochemical tests showed that the SnS2/MoS2 electrode had a specific capacitance of 105.7 F g−1 at a current density of 2.35 A g−1 and it displayed good cyclic stability of 90.4% retention even after 1000 cycles, which indicated that the SnS2/MoS2 was an useful potential electrode material for the application of energy storage and deserves to be further investigated.
1. Introduction
To meet the urgent needs for renewable and sustainable power sources, great attention has been focused on storage devices and energy conversion with ultra fast charge and discharge characteristics, such as supercapacitors.1–3 Supercapacitors are a new energy storage device with high power density, fast charging/discharging rate, super-long cycle life, and excellent cycle stability.4–7Three-dimensional (3D) hierarchical structures, which are comprised of low dimensional nanosheet building block nanostructures, are promising as electrodes.8–11 Because 3D hierarchical structures exhibit the advantages of the pristine building blocks and the improvement property of their secondary architecture. With regard to supercapacitor, the 3D hierarchical structures can facilitate the transportation of electrons and ions and accommodate the volume change of materials in electrochemical reaction process.12–14
Molybdenum disulfide (MoS2), as an important member of two-dimensional (2D) nanomaterials, is composed of three atom layers (S–Mo–S) stacking together via week van der Waals interaction. Up to now, 3D hierarchical structures MoS2, such as flower-like nanostructures,15 hierarchically porous hollow spheres,16–18 have been proved to be effective in improving their electrochemical properties. In general, self-assembled style, which minimize the energy of the reactive system in a spontaneous process, is the simplest synthetic route to obtain 3D hierarchical structures.19 However, the design and electric reactivity of 3D nano-heterostructures have rarely been studied. Kim et al. reported the MoS2 nanostructures synthesized by a hydrothermal route and the specific capacitance of MoS2 was 92.85 F g−1 at a constant discharge current density of 0.5 mA cm−2.20 Ma and co-workers synthesized flower-like MoS2 nanospheres through a hydrothermal method. However, the electrochemical tests showed that the maximum specific capacity was just about 122 F g−1 at 1 A g−1.21 Sun et al. demonstrated polyaniline/MoS2 composites for high-performance supercapacitors and the specific capacitance of the pure MoS2 electrodes was only 98 F g−1 at 1 A g−1.22 However, the rate performances and cycling stabilities of the electrode materials are still unsatisfactory. Ternary sulfides, such as nickel-cobalt sulfides, exhibit an electric conductivity that is much higher than those of single component sulfides. For example, Chen et al. reported that the hierarchical structured NixCo1−xS1.097 electrodes exhibited a remarkable maximum specific capacitance of approximately five times higher than that of the CoS1.097 precursors at a current density of 0.5 A g−1.23 Chen and colleagues proved that the specific capacitance of Ni@Ni3S2 was improved from 89 F g−1 to 122 F g−1 for Ni@Ni1.4Co1.6S2 at a current density of 1 A g−1 with a high loading level (20 mg cm−2). The as-assembled Ni@Ni1.4Co1.6S2//AC showed better cycling stability and coulombic efficiency than Ni@Ni3S2//AC.24 Guan and colleagues fabricated a 3D hierarchical nest-like Ni3S2@NiS with nanorods as building blocks, which was then used as template to prepare Ni3S2@Co9S8 and NiS@NiSe2 electrodes. The specific capacities of Ni3S2@NiS, Ni3S2@Co9S8, and NiS@NiSe2 electrodes were 2440, 6427, and 7717 F g−1, respectively, at a current density of 0.5 A g−1.25 The results indicated that the synergistic effect of double metal ions could enhance the electrochemical performance.
Herein, we report the preparation of MoS2 3D hierarchical structures with grown SnS2 by a simple one-pot approach without using any toxic chemicals. In this nanostructure, Sn ions can be readily embedded onto the flowerlike MoS2 nanosheets through one-top hydrothermal technique. And three-dimensional flowerlike heterostructured SnS2/MoS2 nanosheets act as framework-like substrate to provide a path for ions diffusion. In the nanostructure, SnS2 nanoplates residing in the flowerlike MoS2 nanosheets to prevent the collapse of the MoS2 nanosheets. Thereby, the synergistic effect of MoS2 nanosheets and SnS2 nanoplates is not only able to effectively weaken the agglomerating and restacking problems during the electrochemical reaction, but also able to ensure the high-rate and long-life. The structure, morphology and electrochemical performance of the electrode material were investigated. The as-prepared SnS2/MoS2 electrode exhibits a high capacitance of 105.4 F g−1 at 2.35 A g−1, and also shows excellent cycle stability. The results confirmed that the as-prepared heterostructured SnS2/MoS2 nanostructured electrode exhibits an enhanced electrochemical behavior.
2. Experimental section
2.1 Materials
Sodium molybdate, thiourea, and tin tetrachloride were of analytical grade and used without further purification. In a typical procedure, 2.4 mm Na2MoO4·2H2O, 0.8 mm SnCl4 and 9 mm (NH2)2CS were dissolved in 15 ml of deionized water and 5 ml of absolute ethanol. After stirring for 30 min, the solution was transferred into a 50 ml Teflon-lined stainless steel autoclave and sealed tightly and then heated at 210 °C for 22 h. After cooling naturally, the black precipitates were collected, washed by deionized water several times, and dried at 80 °C for 5 h in a vacuum oven. Finally, the hierarchical heterostructured SnS2/MoS2 were obtained. As a comparison, 3D flower-like MoS2 was obtained, when 2.4 mm Na2MoO4·2H2O and 4.8 mm (NH2)2CS were dissolved in 15 ml of deionized water and 5 ml of absolute ethanol with the other reaction conditions left unchanged.2.2 Characterization
The crystal structure of the obtained SnS2/MoS2 were characterized by X-ray diffraction (XRD) on a PANalytical X' pert PRO instrument using Cu Kα radiation. The morphology of the sample was studied by transmission electron microscope (TEM) on a JEOLJEM-2100 instrument at an accelerating voltage of 80 kV and field-emission scanning electron microscope (FESEM) using a JEOL-JSM6701F instrument at an accelerating voltage of 5 kV. The qualitative information was obtained by X-ray Photoelectron Spectrometer (XPS, VG ESCALAB 210).2.3 Electrochemical measurements
A typical three electrode test cell was used for capacitive performances of the as-prepared sample on a CHI660D (Chenhua, Shanghai, China) electrochemical working station. All of the measurements were carried out in a 1 M KCl aqueous electrolyte solution at room temperature. The working electrode was fabricated by mixing the as-prepared electroactive material, carbon black and poly(tetrafluoroethylene) at a weight ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to form a homogeneous slurry (the total mass of the electrode material was 10 mg), which was pasted onto a piece of nickel foam current collector using a blade. Afterwards, the electrode was dried at 80 °C for 12 h. A saturated calomel electrode (SCE) and platinum sheet were used as the reference and counter electrodes, respectively. Cyclic voltammetry (CV) measurements were carried out in the potential range from −0.9 V to −0.3 V using different scan rates, which was varied from 2 to 20 mV s−1. Galvanostatic charge–discharge (GCD) curves were recorded at different current densities within the potential range from −0.9 V to −0.3 V. Electrochemical impedance spectroscopy (EIS) measurements were performed in the frequency range of 105 to 10−2 Hz.
5 to form a homogeneous slurry (the total mass of the electrode material was 10 mg), which was pasted onto a piece of nickel foam current collector using a blade. Afterwards, the electrode was dried at 80 °C for 12 h. A saturated calomel electrode (SCE) and platinum sheet were used as the reference and counter electrodes, respectively. Cyclic voltammetry (CV) measurements were carried out in the potential range from −0.9 V to −0.3 V using different scan rates, which was varied from 2 to 20 mV s−1. Galvanostatic charge–discharge (GCD) curves were recorded at different current densities within the potential range from −0.9 V to −0.3 V. Electrochemical impedance spectroscopy (EIS) measurements were performed in the frequency range of 105 to 10−2 Hz.
3. Results and discussion
The XRD pattern of the SnS2/MoS2 displays two kinds of diffraction peaks in Fig. 1, besides the diffraction peaks at 13.9° (002), 33.3° (100) and 57.5° (006) reflections assigned to the MoS2 (JCPDS card no. 37-1492),26 all additional ones are well-matched to the SnS2 (JCPDS #23-0677)27 indicating the presence of SnS2 grown with MoS2.The low-magnification SEM image (Fig. 2a) demonstrates that the MoS2 consists of a large quantity of uniform 3D flower-like nanostructures. The flower-like MoS2 has diameters of about 600–800 nm. The high-magnification SEM image (Fig. 2b) reveals that the flower-like nanostructures are composed of intercrossed curved nanoflakes with a thickness of several nanometers. The morphology of the as-synthesized flowerlike MoS2 was further characterized by TEM. As shown in Fig. 2c, TEM image confirmed the existence of flowerlike MoS2 structures, which closely correlates with the results of the SEM measurement. Fig. 3a and b shows the SEM image of the as prepared flower-like SnS2/MoS2 heterostructure. The 3D architecture is helpful to increase the specific area. 3D flower-like heterostructured MoS2/SnS2 facilitates rapid electronic transport in electrode reactions. Furthermore, this structure also enhances the stability of electrochemical performance. Fig. 3c shows the TEM image of SnS2/MoS2 heterostructure. The image reveals a general trend with the sheets of SnS2 homogeneously embedded in MoS2, showing the layered platelets. The mapping analyses on the SnS2/MoS2 (Fig. 3d) reveal the presence of not only Mo and S but also Sn, which confirm the assumption that some SnS2 may be formed in the interior of MoS2. Inductively coupled plasma mass spectroscopy analysis reveals that the ratio of Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn was 3.4
Sn was 3.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
 | ||
| Fig. 3 (a and b) SEM, (c) TEM images of flower-like heterostructured SnS2/MoS2 and (d) corresponding elemental mapping of Mo, Sn, and S. | ||
The obtained SnS2/MoS2 were further characterized by XPS. The high-resolution XPS of Mo 3d exhibits three peaks in Fig. 4a. The peaks at 233.1 and 229.9 eV are Mo 3d3/2 and Mo 3d5/2 binding energies, respectively. These peaks can be attributed to the Mo ion in the +4 oxidation state. The peak 227.1 eV can be ascribed to the 2s binding energy of S in MoS2. The high-resolution of S 2p spectrum showed main doublet located at binding energies of 162.1 and 163.2 eV in Fig. 4b, which can be assigned to the spin–orbit couple S 2p3/2 and S 2p1/2, respectively. These binding energies agrees well with the reported values for the MoS2.28,29 The two strong peaks at around 486.5 and 495 eV are displayed in Fig. 3c. These peaks can be attributed to Sn 3d3/2 and 3d5/2 respectively, which are consistent with the reference data of Sn4+ in SnS2.30
 | ||
| Fig. 4 XPS spectra of the flower-like heterostructured SnS2/MoS2. (a) High-resolution spectra for Mo 3d, (b) high-resolution spectra for S 2p and (c) high-resolution spectra for Sn 3d. | ||
The electrochemical measurement results of the MoS2 and SnS2/MoS2 electrodes were evaluated by cyclic voltammetry (CV). Fig. 5a shows the cyclic voltammograms curves of the SnS2/MoS2 electrode at various scan rates ranging from 2 to 20 mV s−1 in a potential range of −0.9 V to −0.3 V. It can be observed that all the curves exhibit an approximately rectangular shape without any redox peaks which indicates a typical electrical double-layer capacitance feature with fast charging–discharging processes. In addition, the shapes of these CV curves do not significantly change with increasing scan rate from 2 to 20 mV s−1, which reveals the ideal capacitive behavior and good charge collection as well as the facilitated diffusion of K+ in the SnS2/MoS2 electrode.31 Furthermore, the CV curve area increases with the scan rate, indicating that the rates of electric and proton transportation are rapid with respect to the scan rates. The normalized CV of MoS2 nanoflowers at the scan rate of 10 mV s−1 have also been measured and shown for comparison with SnS2/MoS2 in Fig. 5b. Obviously, the SnS2/MoS2 owns larger enclosed area than pure MoS2, suggesting that the former has a larger areal capacitance. This is mainly due to the great contribution of the SnS2/MoS2, which prevents the collapse of the MoS2 nanosheets. Thereby, the synergistic effect of MoS2 nanosheets and SnS2 nanoplates is not only able to effectively weaken the agglomerating and restacking problems, but also able to facilitate rapid electronic transport in electrode reactions.
To further calculate the specific capacitance of the 3D flower-like heterostructured SnS2/MoS2 electrode, the charge/discharge measurements were performed between −0.9 V to −0.3 V at different current densities in 1 M KCl solutions as shown in Fig. 3c. The specific capacitance was calculated by the following equation:
According to the equation, the specific capacitances of the SnS2/MoS2 are 151.9, 127.4, 111.3, and 105.7 F g−1 at 0.24, 0.59, 1.18, and 2.35 A g−1, respectively (Fig. 5c). At low current densities, the inner active sites or the pores of the electrode can be fully accessed and diffused with cations; hence, high specific capacitance values are achieved. The charge/discharge behavior of MoS2 had also been measured and showed in Fig. 5d. The capacitance of the electrode is calculated to be about 145.8, 125.1, 100.3 and 67.3 F g−1 at 0.24, 0.59, 1.18, and 2.35 A g−1, respectively. They own low capacitance (67.3 F g−1 at 2.35 A g−1) compared to SnS2/MoS2 electrode, which deliver an improved capacitance. The enlarged specific capacitance can be attributed to the synergistic effect of two-component heterostructured metal sulfides.
Fig. 6a shows Nyquist plots of the EIS data obtained for the SnS2/MoS2 and MoS2 electrodes at open circuit potential over the frequency range 0.01–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz in 1 M KCl electrolyte solutions. In low frequency area, the Warburg impedance (W), which results from the diffusive resistance of the electrolyte into the interior of the electrode and the ion diffusion into the electrode, is shown by the slope of the curve. The more vertical the curve is, the smaller Warburg impedance is. The slopes of the curve at low frequency area of SnS2/MoS2 electrode is more vertical compared to MoS2, which demonstrates the decreasing of diffusive resistance between the electrode and the electrolyte. In the high frequency area, the semicircle corresponds to the charge-transfer resistance of the electrode and electrolyte interface.32,33 Different from pure MoS2, the semicircle is smaller in SnS2/MoS2 electrode, indicating that the resistance is significant lower. The bulk resistance of the electrochemical system can also be realized from the intersection of the curve at real part Z. From the plots, we can see SnS2/MoS2 electrode shows lower bulk resistance.
000 Hz in 1 M KCl electrolyte solutions. In low frequency area, the Warburg impedance (W), which results from the diffusive resistance of the electrolyte into the interior of the electrode and the ion diffusion into the electrode, is shown by the slope of the curve. The more vertical the curve is, the smaller Warburg impedance is. The slopes of the curve at low frequency area of SnS2/MoS2 electrode is more vertical compared to MoS2, which demonstrates the decreasing of diffusive resistance between the electrode and the electrolyte. In the high frequency area, the semicircle corresponds to the charge-transfer resistance of the electrode and electrolyte interface.32,33 Different from pure MoS2, the semicircle is smaller in SnS2/MoS2 electrode, indicating that the resistance is significant lower. The bulk resistance of the electrochemical system can also be realized from the intersection of the curve at real part Z. From the plots, we can see SnS2/MoS2 electrode shows lower bulk resistance.
 | ||
| Fig. 6 (a) Nyquist plots of MoS2 and SnS2/MoS2 electrodes in 1 M KCl, (b) cycling stability of the MoS2 and SnS2/MoS2 at a current density of 2.35 A g−1. | ||
The cycling stability of the SnS2/MoS2 electrode was investigated by repeating the galvanostatic charge–discharge measurements ranging from −0.9 V to −0.3 V over 1000 cycles at the current density of 2.35 A g−1, as shown in Fig. 6b. The specific capacitance gradually decreases with the cycle number, and the specific capacitance of this electrode still remains at 90.4% after 1000 cycles. Fig. 4b also shows the cycle characteristic of pure MoS2 at a current density of 2.35 A g−1 for up to 500 cycles. After that, it only retains 79% of the initial capacitance with a quite quick decrease. It is clear that the cycle stability of SnS2/MoS2 are greatly improved. The excellent electrochemical performance can be attributed to SnS2/MoS2 heterostructure, which forms an interconnected conducting network, and facilitates rapid electronic transport in electrode reactions.
4. Conclusions
In summary, we have demonstrated a one-step hydrothermal way to fabricate 3D flower-like heterostructured SnS2/MoS2, which had better electrochemical performance compared to the MoS2. The as-prepared SnS2/MoS2 electrode exhibited a high capacitance of 105.4 F g−1 at 2.35 A g−1, and also showed excellent cycle stability. This capacitive behavior mainly resulted from the rapid electronic transport and volume change buffering of SnS2/MoS2 heterostructure during electrochemical measurement. Due to the excellent performance, we believe that the SnS2/MoS2 is a potential promising electrode material for the application of energy storage or conversion with fine electrochemical performance and deserved to be further investigated.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
This work is supported by the Chinese Academy of Sciences and Technology Project (XBLZ-2011-013) and the Technologies R&D Program of Gansu Province (1104FKCA156).References
- M. Winter and R. J. Brodd, What Are Batteries, Fuel Cells, and Supercapacitors, Chem. Rev., 2004, 10, 4245–4270 CrossRef.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Carbon-Based Supercapacitors Produced by Activation of Graphene, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed.
- J. M. Tarascon and M. Armand, Issues and Challenges Facing Rechargeable Lithium Batteries, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- B. E. Conway, V. Birss and J. Wojtowicz, The Role and Utilization of Pseudocapacitance for Energy Storage by Supercapacitors, J. Power Sources, 1997, 66, 1–14 CrossRef CAS.
- D. Pech, M. Brunet, H. Durou, P. Huang, V. Mochalin, Y. Gogotsi, P. L. Taberna and P. Simon, Ultrahigh-power Micrometre-sized Supercapacitors Based on Onion-like Carbon, Nat. Nanotechnol., 2010, 5, 651–654 CrossRef CAS PubMed.
- N. Mahmood, C. Zhang, H. Yin and Y. Hou, Graphene-based Nanocomposites for Energy Storage and Conversion in Lithium Batteries, Supercapacitors and Fuel Cells, J. Mater. Chem. A, 2014, 2, 15–32 CAS.
- P. Simon, Y. Gogotsi and B. Dunn, Where Do Batteries End and Supercapacitors Begin, Science, 2014, 343, 1210–1211 CrossRef CAS PubMed.
- B. Li and Y. Wang, Facile Synthesis and Enhanced Photocatalytic Performance of Flower-like ZnO Hierarchical Microstructures, J. Phys. Chem. C, 2009, 114, 890–896 Search PubMed.
- J. N. Tiwari, R. N. Tiwari and K. S. Kim, Zero-dimensional, One-dimensional, Two-dimensional and Three-dimensional Nanostructured Materials for Advanced Electrochemical Energy Devices, Prog. Mater. Sci., 2012, 57, 724–803 CrossRef CAS PubMed.
- C. Hu, J. Guo and J. Wen, Hierarchical Nanostructure CuO with Peach Kernel-like Morphology as Anode Material for Lithium-Ion Batteries, Ionics, 2013, 19, 253–258 CrossRef CAS.
- J. Zhu, Z. Yin, D. Yang, T. Sun, H. Yu, H. E. Hoster, H. H. Hng, H. Zhang and Q. Yan, Hierarchical Hollow Spheres Composed of Ultrathin Fe2O3 Nanosheets for Lithium Storage and Photocatalytic Water Oxidation, Energy Environ. Sci., 2013, 6, 987–993 CAS.
- J. Wang, G. Du, R. Zeng, B. Niu, Z. Chen, Z. Guo and S. Dou, Porous Co3O4 Nanoplatelets by Self-supported Formation as Electrode Material for Lithium-Ion Batteries, Electrochim. Acta, 2010, 55, 4805–4811 CrossRef CAS PubMed.
- M. H. Park, K. Kim, J. Kim and J. Cho, Flexible Dimensional Control of High-Capacity Li-Ion-Battery Anodes: From 0D Hollow to 3D Porous Germanium Nanoparticle Assemblies, Adv. Mater., 2010, 22, 415–418 CrossRef CAS PubMed.
- A. Esmanski and G. A. Ozin, Silicon Inverse-Opal-Based Macroporous Materials as Negative Electrodes for Lithium Ion Batteries, Adv. Funct. Mater., 2009, 19, 1999–2010 CrossRef CAS PubMed.
- K. J. Huang, L. Wang, Y. J. Liu, H. B. Wang, Y. M. Liu and L. L. Wang, Synthesis of polyaniline/2-dimensional graphene analog MoS2 composites for high-performance supercapacitor, Electrochim. Acta, 2013, 109, 587–594 CrossRef CAS PubMed.
- Z. M. Wan, J. Shao, J. J. Yun, H. Y. Zheng, T. Gao, M. Shen, Q. T. Qu and H. H. Zheng, Core–Shell Structure of Hierarchical Quasi-Hollow MoS2 Microspheres Encapsulated Porous Carbon as Stable Anode for Li-Ion Batteries, Small, 2014, 10, 4975–4981 CrossRef CAS PubMed.
- N. A. Dhas and K. S. Suslick, Sonochemical Preparation of Hollow Nanospheres and Hollow Nanocrystals, J. Am. Chem. Soc., 2005, 127, 2368–2369 CrossRef CAS PubMed.
- M. Wang, G. D. Li, H. Y. Xu, Y. T. Qian and J. Yang, Enhanced Lithium Storage Performances of Hierarchical Hollow MoS2 Nanoparticles Assembled from Nanosheets, ACS Appl. Mater. Interfaces, 2013, 5, 1003–1008 CAS.
- J. Ma, D. Lei, X. Duan, Q. Li, T. Wang, A. Cao, Y. Mao and W. Zheng, Designable Fabrication of Flower-like SnS2 Aggregates with Excellent Performance in Lithium-Ion Batteries, RSC Adv., 2012, 2, 3615–3617 RSC.
- K. Krishnamoorthy, G. K. Veerasubramani, S. Radhakrishnan and S. J. Kim, Supercapacitive properties of hydrothermally synthesized sphere like MoS2 nanostructures, Mater. Res. Bull., 2014, 50, 499–502 CrossRef CAS PubMed.
- X. Zhou, B. Xu, Z. Lin, D. Shu and L. Ma, Hydrothermal Synthesis of Flower-Like MoS2 Nanospheres for Electrochemical Supercapacitors, J. Nanosci. Nanotechnol., 2014, 14, 7250–7254 CrossRef CAS PubMed.
- B. Hu, X. Qin, A. M. Asiri, K. A. Alamry, A. O. AlYoubi and X. Sun, Synthesis of porous tubular C/MoS2 nanocomposites and their application as a novel electrode material for supercapacitors with excellent cycling stability, Electrochim. Acta, 2013, 100, 24–28 CrossRef CAS PubMed.
- Y. Gao, S. Cui, L. Mi, W. Wei, F. Yang, Z. Zheng, H. Hou and W. Chen, Double Metal Ions Synergistic Effect in Hierarchical Metal Sulfide Microflowers for Enhanced Supercapacitor Performance, ACS Appl. Mater. Interfaces, 2015, 7, 4311–4319 CAS.
- L. Mi, W. Wei, S. Huang, S. Cui, W. Zhang, H. Hou and W. Chen, Nest-like Ni@Ni1.4Co1.6S2 Electrode for Flexible High-Performance Rolling Supercapacitors Device Design, J. Mater. Chem. A, 2015 10.1039/C5TA06265A.
- W. Wei, L. Mi, Y. Gao, Z. Zheng, W. Chen and X. Guan, Partial Ion-Exchange of Nickel-Sulfide-Derived Electrodes for High Performance Supercapacitors, Chem. Mater., 2014, 26, 3418–3426 CrossRef CAS.
- H. Luo, C. Xu, D. Zou, L. Wang and T. Ying, Hydrothermal synthesis of hollow MoS2 micro-spheres in ionic liquids/water binary emulsions, Mater. Lett., 2008, 62, 3558–3560 CrossRef CAS PubMed.
- M. K. Jana, H. B. Rajendra, A. J. Bhattacharyya and K. Biswas, Green ionothermal synthesis of hierarchical nanostructures of SnS2 and their Li-ion storage properties, CrystEngComm, 2014, 16, 3994–4000 RSC.
- W. J. Li, E. W. Shi, J. M. Ko, Z. Chen, H. Ogino and T. J. Fukuda, Hydrothermal synthesis of MoS2 nanowires, Cryst. Growth Des., 2003, 250, 418–422 CAS.
- H. Lin, X. Chen, H. Li, M. Yang and Y. Qi, Hydrothermal synthesis and characterization of MoS2 nanorods, Mater. Lett., 2010, 64, 1748–1750 CrossRef CAS PubMed.
- Y. Zhang, J. Li, M. Zhang and D. D. Dionysiou, Size-tunable hydrothermal synthesis of SnS2 nanocrystals with high performance in visible light-driven photocatalytic reduction of aqueous Cr(VI), Environ. Sci. Technol., 2011, 45, 9324–9331 CrossRef CAS PubMed.
- J. Li, Q. M. Yang and I. Zhitomirsky, Nickel foam-based manganese dioxide–carbon nanotube composite electrodes for electrochemical supercapacitors, J. Power Sources, 2008, 185, 1569–1574 CrossRef CAS PubMed.
- H. R. Oswald, J. R. Gunter and E. J. Dubler, Topotactic decomposition and crystal structure of white molybdenum trioxide-monohydrate: prediction of structure by topotaxy, Solid State Chem., 1975, 13, 330–338 CrossRef CAS.
- T. Nagasawa and K. Kobayashi, Paracrystalline Structure of Polymer-Crystal Lattice Distortion Induced by Electron Irradiation, J. Appl. Phys., 1970, 41, 4276–4284 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |