An ICT based ultraselective and sensitive fluorescent probe for detection of HClO in living cells†
Yongkang Yuea,
Fangjun Huob,
Caixia Yin*a,
Jianbin Chaob,
Yongbin Zhang†b and
Xing Weia
aInstitute of Molecular Science, Shanxi University, Taiyuan 030006, China. E-mail: yincx@sxu.edu.cn; Fax: +86 351 7011022; Tel: +86 351 7011022
bResearch Institute of Applied Chemistry, Shanxi University, Taiyuan 030006, China
First published on 9th September 2015
Abstract
An ICT based ultraselective and sensitive probe for colorimetric and fluorescent detection of HClO via oxidative cleavage of an alkene linker to epoxide and then to aldehydes was developed through the conjugation of pyridinium with vanilline.
Hypochlorite (ClO−) and its protonated form (HClO) as strong oxidizing agents are widely employed in various organic syntheses, disinfectants and bleaches.1–3 In biological system, hypochlorous acid which is produced from peroxidation of chloride ions with the catalysis of heme enzyme myeloperoxidase (MPO) acts as an essential antimicrobial agent in organisms.4 However, excess HClO can cause tissue damage and diseases such as hepatic ischemia–reperfusion injury, atherosclerosis, lung injury, rheumatoid and cardiovascular diseases, neuron degeneration, arthritis, and cancer.5–9 Because of the biological and environmental importance of hypochlorous acid, chemists have made great efforts to measure the content of HClO as accurate as possible. Anteriorly, instrumental methods including potentiometry, polarography and coulometric have been applied in the hypochlorous acid detect.10–12 Recently, optical probes have attracted significant interest because of its advantages such as high sensitivity and selectivity, speed, friendliness compared with traditional instrumental methods.
Up to now, the reported probes for HClO detection mainly include p-methoxyphenol oxidation to p-benzoquinone,13 oxime oxidation to aldehydes,14 the chlorination of thiols and amines,15 p-aminophenol analog oxidation,16 thioether to sulfonate and selenide to selenoxide transformations,17,18 the cleavage of carbon–carbon double bonds,19 oxidative processes in metal complexes and others20–22 which have made great progresses. However, most of them might encounter the problems like poor water solubility and lineation, low quantum yield, tardy responses and interrogative selective. What's more, because of the strong oxidizability of HClO, most of the oxidation products displayed uncertain or unstable structure which obfuscated the detection mechanisms. To improve those imperfect factors, probes for HClO with sufficient properties still should be developed.
As a well know electron-withdrawing group, 1,4-dimethylpyridin-1-ium iodide moiety is always employed to improve the water solubility of the probe.23 At the same time, systems with electron donor and acceptor moieties connected by conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond would generally exhibit non fluorescence through intramolecular charge transfer (ICT) process. However, the broken of the conjugation system by HClO would induce distinct fluorescent changes because of the interdiction of ICT process.4,24–26 With these considerations in mind, we designed and synthesized a new colorimetric and fluorescent probe with a vanillina moiety (electron donor) and methylpyridin-1-ium iodide moiety (electron acceptor) based on ICT process. This new fluorescent probe (probe 1 in Scheme 1) exhibit excellent turn-on fluorescent emission when encounter with HClO within 10 s. With the characterization of the oxidative product, we confirmed the detection mechanism demonstrably. Furthermore, this probe was successfully applied in fluorescent imaging in living cells.
C bond would generally exhibit non fluorescence through intramolecular charge transfer (ICT) process. However, the broken of the conjugation system by HClO would induce distinct fluorescent changes because of the interdiction of ICT process.4,24–26 With these considerations in mind, we designed and synthesized a new colorimetric and fluorescent probe with a vanillina moiety (electron donor) and methylpyridin-1-ium iodide moiety (electron acceptor) based on ICT process. This new fluorescent probe (probe 1 in Scheme 1) exhibit excellent turn-on fluorescent emission when encounter with HClO within 10 s. With the characterization of the oxidative product, we confirmed the detection mechanism demonstrably. Furthermore, this probe was successfully applied in fluorescent imaging in living cells.
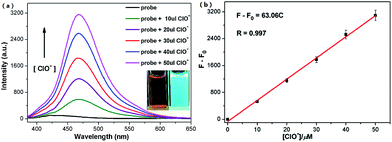
To exam the detection property of probe 1 towards HClO, we firstly measured the titration experiment in DMSO–HEPES buffer (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) at 25 °C. As shown in Fig. 1a, probe 1 (50 μM) exhibited non fluorescent emission with the excitation at 373 nm in DMSO–HEPES buffer (3
1, v/v, pH 7.4) at 25 °C. As shown in Fig. 1a, probe 1 (50 μM) exhibited non fluorescent emission with the excitation at 373 nm in DMSO–HEPES buffer (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4). However, the addition of HClO induced a significant fluorescent emission at 468 nm gradually and further peaked with a fifty-fold fluorescent intensity increasing (Φf = 0.341 with quinine sulfate as reference) when the concentration of HClO reached 50 μM. The corresponding UV-vis spectral changes were also studied (Fig. S5a†). The solution of 25 μM probe 1 in DMSO–HEPES buffer (3
1, v/v, pH 7.4). However, the addition of HClO induced a significant fluorescent emission at 468 nm gradually and further peaked with a fifty-fold fluorescent intensity increasing (Φf = 0.341 with quinine sulfate as reference) when the concentration of HClO reached 50 μM. The corresponding UV-vis spectral changes were also studied (Fig. S5a†). The solution of 25 μM probe 1 in DMSO–HEPES buffer (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) had two broad peaks centered at 470 nm and 345 nm. With addition of HClO (0–20 μM) to the above system, the original absorption intensity at both 470 nm and 345 nm decreased and the new peak at 269 nm increased synchronously and a well-defined isoabsorptive point was noted at 310 nm. Meanwhile, the color of the system changed from claybank to pale yellow. The ultraviolet absorption changes of the detection process mean the broken of the conjugation system. The kinetic analysis of 1 towards 10 equiv. of HClO displayed that the reaction was complete within 10 s (Fig. S6†). These fast and distinct responses both in fluorescent emission and color changes promoted probe 1 to been used as a colorimetric and fluorescent probe for HClO.
1, v/v, pH 7.4) had two broad peaks centered at 470 nm and 345 nm. With addition of HClO (0–20 μM) to the above system, the original absorption intensity at both 470 nm and 345 nm decreased and the new peak at 269 nm increased synchronously and a well-defined isoabsorptive point was noted at 310 nm. Meanwhile, the color of the system changed from claybank to pale yellow. The ultraviolet absorption changes of the detection process mean the broken of the conjugation system. The kinetic analysis of 1 towards 10 equiv. of HClO displayed that the reaction was complete within 10 s (Fig. S6†). These fast and distinct responses both in fluorescent emission and color changes promoted probe 1 to been used as a colorimetric and fluorescent probe for HClO.
In order to investigate the sensitivity of probe 1 to HClO, the working curve was further measured upon treatment of 50 μM probe 1 with various concentrations of HClO (0–50 μM) in DMSO–HEPES buffer (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4). As shown in Fig. 1b, a linear calibration graph of the responses of the relative fluorescent intensity (F − F0) at 468 nm to the HClO concentrations from 0 to 50 μM could be observed, which means that probe 1 could be potentially employed to detect HClO quantitatively. The detection limit, based on the definition by IUPAC (CDL = 3 Sb m−1), was found to be 0.068 μM from 10 blank solutions. This result demonstrated probe 1 to be a highly sensitive probe for HClO compared with other reported HClO chemosensors (Table 1).27,28,29
1, v/v, pH 7.4). As shown in Fig. 1b, a linear calibration graph of the responses of the relative fluorescent intensity (F − F0) at 468 nm to the HClO concentrations from 0 to 50 μM could be observed, which means that probe 1 could be potentially employed to detect HClO quantitatively. The detection limit, based on the definition by IUPAC (CDL = 3 Sb m−1), was found to be 0.068 μM from 10 blank solutions. This result demonstrated probe 1 to be a highly sensitive probe for HClO compared with other reported HClO chemosensors (Table 1).27,28,29
To value the selectivity of probe 1 for HClO, various analytes including ClO−, H2O2, 1O2, NO, O2˙−, HO˙, ClO2−, IO4−, ONOO−, ROO˙, CN−, HS−, HSO3− and Cys were measured in a solution of 50 μM 1 in DMSO–HEPES buffer (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4). Delightfully, even 100 equiv. of other anions and Cys did not induce any fluorescent increase (Fig. 2a) of the system except the introduction of HClO. At the same time, the competition experiments were measured to detect HClO by probe 1 with the presence of other anions and Cys. As shown in Fig. 2b, all the competing anions did not interfere the detection of HClO. This result displayed highly selectivity of probe 1 towards HClO over other analytes mentioned above.
1, v/v, pH 7.4). Delightfully, even 100 equiv. of other anions and Cys did not induce any fluorescent increase (Fig. 2a) of the system except the introduction of HClO. At the same time, the competition experiments were measured to detect HClO by probe 1 with the presence of other anions and Cys. As shown in Fig. 2b, all the competing anions did not interfere the detection of HClO. This result displayed highly selectivity of probe 1 towards HClO over other analytes mentioned above.
To further confirm the detection mechanism, we measured the NMR titration experiment through adding HClO to a solution of probe 1 in DMSO-d6. Compared with the 1H NMR spectrogram of probe 1, the signal of original alkene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C proton at 7.65 ppm and 7.99 ppm disappeared gradually with the addition of HClO (Fig. S3†). Those signal changes indicated the cleavage of the alkene linker in probe 1 caused by HClO. Moreover, the peak at 285.46 corresponding to [2]+ and 178.92 corresponding to [3 − H]− and 122.83 corresponding to [4]+ in the MS spectrums further proved the mechanism mentioned above (Fig. S3†). Thus, the sensing mechanism of probe 1 towards HClO was based on the cleavage of the alkene linker to epoxide and then to aldehydes as shown in Scheme 2.
C proton at 7.65 ppm and 7.99 ppm disappeared gradually with the addition of HClO (Fig. S3†). Those signal changes indicated the cleavage of the alkene linker in probe 1 caused by HClO. Moreover, the peak at 285.46 corresponding to [2]+ and 178.92 corresponding to [3 − H]− and 122.83 corresponding to [4]+ in the MS spectrums further proved the mechanism mentioned above (Fig. S3†). Thus, the sensing mechanism of probe 1 towards HClO was based on the cleavage of the alkene linker to epoxide and then to aldehydes as shown in Scheme 2.
To value the practical utilities of probe 1, we further measured the cell experiments to detect exogenous HClO. Firstly, the cytotoxicity experiments displayed that probe 1 had only minimal cytotoxicity (Fig. S7†). As shown in Fig. 3a, HepG2 cells displayed non fluorescence when incubated with 30 μM probe 1 only for 30 min at 37 °C. However, incubating with HClO (10 μM, 20 μM and 30 μM respectively) would induce significant fluorescent emission of HepG2 cells which were preincubated with probe 1 (30 μM) (Fig. 3b–d). These results displayed excellent membrane permeability of probe 1 and further demonstrated that probe 1 could be applied to detect HClO in living cells.
In conclusion, we developed a new probe for colorimetric and fluorescent detection of HClO in aqueous solution. Based on the cleavage of an alkene linker in probe 1 to epoxide and then to aldehydes mechanism which break the ICT process with in the pyridinium (acceptor) and vanilline (donor) moieties, probe 1 displayed ultraselective and sensitive optical responses to HClO. Further cellular experiments displayed that probe 1 could be applied in bioimaging.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 21472118), the Shanxi Province Foundation for Returnee (No. 2012-007), the Taiyuan Technology star special (No. 12024703), the Program for the Top Young and Middle-aged Innovative Talents of Higher Learning Institutions of Shanxi (TYMIT, No. 2013802), talents Support Program of Shanxi Province (No. 2014401).Notes and references
- J. Skarzewski and R. Siedlecka, Org. Prep. Proced. Int., 1992, 24, 623 CrossRef CAS.
- Y. K. Yue, C. X. Yin, F. J. Huo, J. B. Chao and Y. B. Zhang, Sens. Actuators, B, 2014, 202, 551 CrossRef CAS.
- K. Vijayaraghavan, T. K. Ramanujam and N. Balasubramanian, Waste Management, 1999, 19, 319 CrossRef CAS.
- M. T. Sun, H. Yu, H. J. Zhu, F. Ma, S. Zhang, D. J. Huang and S. H. Wang, Anal. Chem., 2014, 86, 671 CrossRef CAS PubMed.
- Y. W. Yap, M. Whiteman and N. S. Cheung, Cell. Signal., 2007, 19, 219 CrossRef CAS PubMed.
- L. Gebicka and E. Banasiak, Toxicol. In Vitro, 2012, 26, 924 CrossRef CAS PubMed.
- T. G. Favero and D. Colter, J. Appl. Physiol., 1998, 84, 425 CAS.
- J. G. Martin, H. R. Campbell, H. Iijima, D. Gautrin, J. L. Malo, D. H. Eidelman, Q. Hamid and K. Maghni, Am. J. Respir. Crit. Care Med., 2003, 168, 568 CrossRef PubMed.
- C. H. Sam and H. K. Lu, J. Dent. Sci., 2009, 4, 45 CrossRef.
- S. Thiagarajan, Z. Y. Wu and S. M. Chen, J. Electroanal. Chem., 2011, 661, 322 CrossRef CAS.
- A. F. Krivis and E. S. Gazda, Anal. Chem., 1967, 39, 226 CrossRef CAS.
- L. C. Adam and G. Gordon, Anal. Chem., 1995, 67, 535 CrossRef CAS.
- W. J. Zhang, C. Guo, L. H. Liu, J. G. Qin and C. L. Yang, Org. Biomol. Chem., 2011, 9, 5560 CAS.
- G. F. Wu, F. Zeng and S. Z. Wu, Anal. Methods, 2013, 5, 5589 RSC.
- Q. L. Xu, K. A. Lee, S. Y. Lee, K. M. Lee, W. J. Lee and J. Y. Yoon, J. Am. Chem. Soc., 2013, 135, 9944 CrossRef CAS PubMed.
- T. Guo, L. Cui, J. N. Shen, R. Wang, W. P. Zhu, Y. F. Xu and X. H. Qian, Chem. Commun., 2013, 49, 1862 RSC.
- L. X. Lu, J. Zhang and X. R. Yang, Sens. Actuators, B, 2013, 184, 189 CrossRef CAS.
- G. H. Cheng, J. L. Fan, W. Sun, J. F. Cao, C. Hu and X. J. Peng, Chem. Commun., 2014, 50, 1018 RSC.
- J. S. Park, H. J. Kim, Y. D. Choi and Y. M. Kim, Analyst, 2013, 138, 3368 RSC.
- H. Zhu, J. L. Fan, J. Y. Wang, H. Y. Mu and X. J. Peng, J. Am. Chem. Soc., 2014, 136, 12820 CrossRef CAS PubMed.
- Y. R. Zhang, X. P. Chen, J. Shao, J. Y. Zhang, Q. Yuan, J. Y. Miao and B. X. Zhao, Chem. Commun., 2014, 50, 14241 CAS.
- S. T. Manjare, J. Kim, Y. Lee and D. G. Churchill, Org. Lett., 2014, 16, 520 CrossRef CAS PubMed.
- Y. H. Zhang, J. J. Wang, P. F. Jia, X. Q. Yu, H. Liu, X. Liu, N. Zhao and B. B. Huang, Org. Biomol. Chem., 2010, 8, 4582 CAS.
- N. Zhao, M. Li, Y. L. Yan, J. W. Y. Lam, Y. L. Zhang, Y. S. Zhao, K. S. Wong and B. Z. Tang, J. Mater. Chem. C, 2013, 1, 4640 RSC.
- M. Busi, B. Cantadori, F. Boccini, R. de Zorzi, S. Geremia and E. Dalcanale, Eur. J. Org. Chem., 2011, 2629 CrossRef CAS.
- W. G. Yang, Y. Wong, O. T. W. Ng, L. P. Bai, D. W. J. Kwong, Y. Ke, Z. H. Jiang, H. W. Li, K. K. L. Yung and M. S. Wong, Angew. Chem., Int. Ed., 2012, 51, 1804 CrossRef PubMed.
- L. L. Long, Y. J. Wu, L. Wang, A. H. Gong, F. L. Hu and C. Zhang, Chem. Commun., 2015, 51, 10435–10438 RSC.
- W. Zhang, W. Liu, P. Li, J. Q. Kang, J. Y. Wang, H. Wang and B. Tang, Chem. Commun., 2015, 51, 10069 RSC.
- J. Y. Kim and Y. M. Kim, Analyst, 2014, 139, 2986 RSC.
- L. Yuan, L. Wang, B. K. Agrawalla, S. J. Park, H. Zhu, B. Sivaraman, J. J. Peng, Q. H. Xu and Y. T. Chang, J. Am. Chem. Soc., 2015, 137, 5930 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR, 13C NMR, ESI-MS, crystallographic data and supplementary figures. CCDC 1417924. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra16097a |
| This journal is © The Royal Society of Chemistry 2015 |