Synthesis of amidoximated graphene oxide nanoribbons from unzipping of multiwalled carbon nanotubes for selective separation of uranium(VI)
Yun Wangab,
Zhengshang Wanga,
Ran Anga,
Jijun Yanga,
Ning Liu*a,
Jiali Liaoa,
Yuanyou Yanga and
Jun Tang*a
aKey Laboratory of Radiation Physics and Technology, Ministry of Education, Institute of Nuclear Science and Technology, Sichuan University, Chengdu 610064, China. E-mail: tangjun@scu.edu.cn; nliu720@scu.edu.cn; Fax: +86-28-85416215, +86-28-85412374; Tel: +86-28-85416215, +86-28-85416858
bSchool of Chemistry, Biological and Materials Science, East China Institute of Technology, Nanchang 330013, Jiangxi, China
First published on 5th October 2015
Abstract
A kind of uranium-selective sorbent has been studied using graphene oxide nanoribbons (GONRs) from the unzipping of multiwalled carbon nanotubes as a solid matrix and amidoxime (AO) as a functional group. Amidoxime-functionalized GONRs (AOGONRs) were successfully prepared by chemical grafting technology and characterized by scanning electron microscopy, X-ray powder diffraction, Fourier transform infrared spectroscopy, elemental analysis, thermogravimetric analysis and X-ray photoelectron spectroscopy. The as-prepared AOGONRs were applied to adsorb U(VI) from aqueous solutions and exhibited a high sorption capacity towards U(VI) due to the strong chelation of AO to U(VI). It can be noted that the uranium sorption on the AOGONRs was pH-dependent, ionic strength-independent, fast, endothermic, spontaneous and a pseudo-second order process. The U(VI) sorption amount reached up to 2.112 mmol g−1 (502.6 mg g−1) at pH = 4.5 and T = 298 K. The sorption study performed in a simulated nuclear industry effluent demonstrated that the new sorbent had a desirable selectivity towards U(VI) ions over a range of competing metal ions. The results suggest that AOGONRs may be a potential and suitable candidate for the separation of U(VI) from various uranium-containing water samples.
Introduction
Graphene,1 a 2D single atomic layer of sp2 carbon, has been regarded as a star material owing to its unique physical and chemical properties, such as its high thermal conductivity, large surface area, and good thermal stability in comparison to the conventional allotropes of carbon.2–4 In the past few years, graphene has attracted much interest regarding its application in environmental science. In particular, graphene oxide (GO), with abundant oxygen-bearing groups on its surface, is much more hydrophilic than graphene itself, and thus can efficiently capture metal ions through the sharing of an electron pair of the oxygen atom.5 GO has been proven to be non-toxic and biodegradable and the adsorption/desorption of metal ions could be easily performed, which makes it suitable to be applied in environmental science.6–9 However, the high cost of GO greatly limits its large-scale application, and the sorption capacity of metal ions on GO needs to be further improved. Therefore, exploring an alternative graphene-based material with a higher sorption capacity and lower cost for the sorption of metal ions is imperative.In particular, nanometer-wide ribbons of graphene, namely graphene nanoribbons (GNRs), is one of the most promising materials for this purpose. Several approaches have been developed to synthesize GNRs, such as lithographic pattering, sonochemical methods, chemical vapor deposition and unzipping of multiwalled carbon nanotubes (MWCNTs).10,11 Among these methods, the oxidative chemical unzipping of MWCNTs using sulfuric acid (H2SO4) and potassium permanganate (KMnO4) developed by Tour’s group offers a unique way for the bulk production of GNRs.12 Since the unzipping process is oxidative and similar to the process for the production of GO, these nanoribbons are termed graphene oxide nanoribbons (GONRs) and possess oxygen-containing functional groups such as –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –COOH and –OH at the edges and surfaces. These functional groups are essential for the capture of metal ions, and also facilitate the following functionalization process. This provides a promising sorbent to remove metal ions from aqueous solutions. However, to the best of our knowledge, few studies have been reported in environmental applications for GONRs. Recently, our group firstly used GONRs to remove uranium from wastewater and an impressive maximum sorption amount of 394.1 mg g−1 was achieved.13 But the GONRs still showed poor selectivity toward nuclides of interest, and the sorption capacity was not high enough. Therefore, further investigation to improve the selectivity and loading capacity of GONRs towards uranium is of great importance.
O, –COOH and –OH at the edges and surfaces. These functional groups are essential for the capture of metal ions, and also facilitate the following functionalization process. This provides a promising sorbent to remove metal ions from aqueous solutions. However, to the best of our knowledge, few studies have been reported in environmental applications for GONRs. Recently, our group firstly used GONRs to remove uranium from wastewater and an impressive maximum sorption amount of 394.1 mg g−1 was achieved.13 But the GONRs still showed poor selectivity toward nuclides of interest, and the sorption capacity was not high enough. Therefore, further investigation to improve the selectivity and loading capacity of GONRs towards uranium is of great importance.
Amidoxime (AO) groups, one of the most effective chelating functional groups, have attracted special attention for the removal of uranium from various aqueous solutions because of their high selectivity and affinity for uranium.14–17 Carbon-based materials with AO groups have been widely used as sorbents for the removal of uranyl ions.18–23 For instance, amidoxime-grafted hydrothermal carbon showed a uranium sorption capacity as high as 466 mg g−1 and demonstrated stronger selectivity in acidic medium.24 Amidoximated magnetite/graphene oxide composites were applied to adsorb uranium from aqueous solutions and a maximum sorption capacity of 284.9 mg g−1 was found.25 Our group grafted amidoxime groups onto MWCNTs by using plasma techniques to selectively separate uranium from simulated nuclear industrial effluents, and an optimum sorption capacity of 145 mg g−1 for U(VI) was obtained.26 In this work, amidoximated GONRs (AOGONRs) were synthesized using GONRs as the solid matrix and DAMN as a precursor of the AO group. Then, the behavior of the new sorbent AOGONRs for the removal of U(VI) from uranium-containing aqueous solutions was studied in detail. Moreover, the possible sorption mechanism of the sorption process was explored.
Experimental
Chemicals and reagents
MWCNTs were obtained from the Chengdu Institute of Organic Chemistry of the Chinese Academy of Sciences.Chemicals and reagents such as N,N-dimethylformamide (DMF), tetrahydrofuran (THF), 4-dimethylaminopyridine (DMAP), ethanol, dichloromethane, K2CO3, NaOH, HNO3, and hydroxylamine hydrochloride (NH2OH·HCl) used in this research were purchased from Chengdu Kelong Chemical Reagent Co., Ltd. (China). Diaminomaleonitrile (DAMN) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) were purchased from Energy Chemical Co., Ltd. (China). All metal oxides and nitrates were purchased from Aladdin Chemistry Co., Ltd. (China). All reagents were of AR grade and were used without further purification.
Preparation of GONRs
GONRs were fabricated by the longitudinal unzipping of MWCNTs.12 Typically, 1 g of MWCNTs was immersed in 150 mL of sulfuric acid (H2SO4, 98%) for 6 h at room temperature. Then, 500 wt% of KMnO4 was added to the reaction mixture which was then stirred for 1 h at room temperature. The mixture was then heated to 55 °C for 30 min. The reaction temperature was then raised to 70 °C, and then cooled to room temperature. Thereafter the mixture was poured onto 400 mL of ice containing 5 mL of 30% H2O2 solution and then filtered through a PTFE membrane (5.0 μm). The residue was washed with deionized water, followed by centrifugation and a dialysis process (10 K, MWCO, for one week or more) to remove the inorganic acid and impurities. Finally, the mixture was filtered through a PTFE membrane and the filtered product was dried in a vacuum oven at 60 °C for 24 h. The prepared samples were denoted as GONRs.Preparation of AOGONRs
The as-prepared GONRs (2.0 g) and EDCI (2.2 g) were dispersed in THF (60 mL). Then 2.0 g of DAMN and 0.12 g of DAMP were dissolved in 10 mL DMF and added to the reaction system. The mixture was sonicated for 1 h to get a homogeneous colloidal suspension and then refluxed for 24 h with stirring to graft DAMN onto the GONRs. After the condensation reaction was terminated, the resultant mixture was filtered and washed with ethanol and deionized water until the filtrate became colorless. Then the resulting solid (GONRs–DAMN) was washed by dichloromethane to remove DMF, and finally washed thoroughly with ethanol and dried in a vacuum oven at 60 °C. GONRs–DAMN was then treated with 1.0 g K2CO3 and 1.5 g NH2OH·HCl in a 50/50 H2O–C2H5OH solution (pH 8.5) for 10 h at 80 °C in a closed flask. Finally the mixture was filtered, the residue was separated, rinsed and dried at 60 °C in a vacuum overnight, and the final AOGONRs product was obtained. The schematic presentation of the synthesis process is shown in Fig. 1.Characterization
The samples were characterized by scanning electron microscopy (SEM), X-ray powder diffraction (XRD), Fourier transform infrared (FT-IR) spectroscopy, elemental analysis, thermogravimetric analysis (TGA) and X-ray photoelectron spectroscopy (XPS). The SEM images were taken on a Hitachi S-4800 microscope. The XRD patterns were measured on a DX-2700 diffractometer with a Cu Kα source. FT-IR spectra were obtained from a Nicolet 6700 spectrometer. The contents of carbon, hydrogen, and nitrogen in the samples were determined by an elemental analyzer (Carlo-Erba 1106, Italy). TGA was carried out on a TG 209 F1 apparatus in a N2 atmosphere from room temperature to 800 °C at a rate of 10 °C min−1. The XPS spectra were recorded on a Kratos AXIS ULTRADLD electron spectrometer with a multidetection analyzer using an Al Kα X-ray source (1486.6 eV) at 10 kV and 5 mA under 10−8 Pa residual pressure.Sorption experiments
Batch experiments were carried out to study the sorption behavior of AOGONRs towards U(VI). A certain amount of sorbent was added into Erlenmeyer flasks with 25 mL of either a pure U(VI) solution or a multi-ion solution (simulated nuclear industrial effluent sample) containing 12 co-existing cations (Table 1). The pH of the solution was adjusted using HNO3 and NaOH solutions and measured on a digital pH-meter. After being shaken for a specific time, the solutions were centrifuged at 9000 rpm for 30 min, and then the supernatant was filtered using 0.45 μm membrane filters. The initial and the residual concentration of uranium were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES, Optima 8000, USA).| Coexistent ion | Added as | Reagent purity |
|---|---|---|
| UO22+ | UO2(NO3)2·6H2O | Standard reagent |
| La3+ | La(NO3)3·6H2O | 99.9% metal basis |
| Ce3+ | Ce(NO3)3·6H2O | 99.99% metal basis |
| Nd3+ | Nd(NO3)3·6H2O | AR |
| Sm3+ | Sm(NO3)3·6H2O | AR |
| Gd3+ | Gd(NO3)3·6H2O | AR |
| Mn2+ | MnO | 99.5% |
| Co2+ | Co(NO3)2·6H2O | 99.99% metal basis |
| Ni2+ | Ni(NO3)2·6H2O | Spectrum pure |
| Zn2+ | Zn(NO3)2·6H2O | 99.99% metal basis |
| Sr2+ | Sr(NO3)2 | 99.99% metal basis |
| Ba2+ | Ba(NO3)2 | 99.999% |
The sorption amount qe (mmol g−1) and distribution coefficient Kd (mL g−1) were calculated by eqn (1) and (2),
 | (1) |
 | (2) |
Desorption and reusability studies
To carry out the desorption experiments, the solid residue of the sorption experiments was thoroughly rinsed with deionized water and dispersed in 25 mL HCl solution of different concentrations, and allowed to equilibrate for 2 h. After solid–liquid separation, the remaining U(VI) concentration in the supernatant was measured to evaluate the desorption percentage. To determine the reusability, consecutive sorption–desorption cycles were repeated 5 times with the same sorbent using fresh U(VI) solution (0.25 mmol L−1, pH = 4.5) at 298 K. Regeneration of the sorbent was carried out using 0.5 mol L−1 HCl. All experimental series were performed at least in duplicate.Results and discussion
Characterizations
The morphologies of MWCNTs, GONRs, GONRs–DAMN and AOGONRs were observed by SEM, as shown in Fig. 2. The pristine MWCNTs (Fig. 2a) are curved and entangled and have diameters ranging from 10–50 nm and lengths in the micrometer range. As can be seen in Fig. 2b, the MWCNTs were completely unzipped, resulting in complex, wavy-structured GONR strips. The typical width of the strips lies in the 30–100 nm range, while the length of the strips lies in the micrometer range. The simple oxidative process can generate a near 100% yield of nanoribbon structures by the lengthwise cutting and unraveling of the MWCNT side walls.27 Compared with the GONRs, no obvious morphological changes were observed on the surfaces of the functionalized GONRs (Fig. 2c and d), but they are more aggregated with each other, which may be attributed to the strong interaction between the GONRs and functional groups such as AO.28Further structural information and the crystal planes of MWCNTs, GONRs, GONRs–DAMN and AOGONRs were characterized by XRD. As shown in Fig. 3, the MWCNTs show a typical peak at 2θ = 26.0°, corresponding to an interlayer spacing of 3.4 Å, while the GONRs show a predominant peak at 2θ = 10.0°, corresponding to a d-spacing of 8.6 Å. This result indicates the formation of GONRs from the unzipping of MWCNTs.29 However, in the XRD patterns of GONRs–DAMN and AOGONRs, the peak at 2θ = 10.0° disappears and a new peak also appears at 26.0°. The decrease in interplanar spacing indicates that the structure of the GONRs has changed and the graphene layers of the GONRs have been compacted after functionalization.30 This change can be attributed to the grafted functional groups strengthening the interaction between the GONRs,31 which was also confirmed by SEM.
For the characterization of the major surface groups, FT-IR studies were carried out (Fig. 4). In the spectrum of the MWCNTs, the bands at 3440 and 2920 cm−1 can be assigned to the O–H stretching vibration arising from the surface hydroxyl groups and the saturated C–H vibration, respectively. The peak at 1578 cm−1 results from stretching vibrations of isolated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds and is partly superposed by a relatively strong water band at about 1634 cm−1 originating from residual humidity in the pellet.32 Compared to the spectrum of MWCNTs, a new peak at 1714 cm−1 appears in the spectrum of the GONRs, which corresponds to the stretch of carboxylic (–COOH) groups.5,7 In the spectrum of GONRs–DAMN, the intensity of the 1714 cm−1 band (C
C double bonds and is partly superposed by a relatively strong water band at about 1634 cm−1 originating from residual humidity in the pellet.32 Compared to the spectrum of MWCNTs, a new peak at 1714 cm−1 appears in the spectrum of the GONRs, which corresponds to the stretch of carboxylic (–COOH) groups.5,7 In the spectrum of GONRs–DAMN, the intensity of the 1714 cm−1 band (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) decreases sharply, and two new frequencies appear at 1468 cm−1 and 2206 cm−1, separately, belonging to C–N and C
O) decreases sharply, and two new frequencies appear at 1468 cm−1 and 2206 cm−1, separately, belonging to C–N and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching vibrations, clearly supporting the presence of DAMN.24 In the spectrum of AOGONRs, the absorption band of the cyano group at 2206 cm−1 disappeared while a new band at 942 cm−1 corresponding to the N–O stretching vibrations of amidoxime groups was observed, indicating the consumption of cyano groups and the formation of the oxime groups after amidoximation.22,25
N stretching vibrations, clearly supporting the presence of DAMN.24 In the spectrum of AOGONRs, the absorption band of the cyano group at 2206 cm−1 disappeared while a new band at 942 cm−1 corresponding to the N–O stretching vibrations of amidoxime groups was observed, indicating the consumption of cyano groups and the formation of the oxime groups after amidoximation.22,25
To further confirm the existence of cyano group and amidoxime groups, elemental analysis was carried out and the results are shown in Table 2. Only a little nitrogen is detected in the GONRs, which could be ascribed to N-containing impurities. An obvious increase in nitrogen content was observed for GONRs–DAMN, indicating that DAMN was grafted onto the surface of the GONRs, as supported by the previous FT-IR analysis. The amounts of DAMN are calculated from the increment of the content of nitrogen to be about 3.11 mmol g−1. It is also found that the carbon content decreased with the increase in nitrogen content going from GONRs–DAMN to AOGONRs, which might be attributed to the transformation of cyano groups to amidoxime groups by the treatment of hydroxylamine in an alkaline medium.24
| Samples | C% | H% | N% |
|---|---|---|---|
| MWCNTs | 98.92 | 1.03 | 0.00 |
| GONRs | 62.58 | 1.69 | 0.43 |
| GONRs–DAMN | 59.12 | 2.81 | 9.14 |
| AOGONRs | 48.27 | 3.23 | 9.53 |
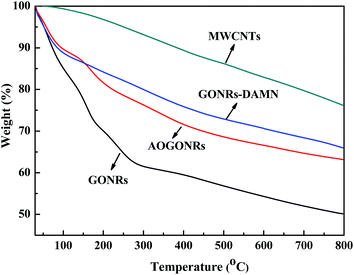
Fig. 5 shows the TGA curves of MWCNTs, GONRs, GONRs–DAMN and AOGONRs. The weight losses of the four materials can be divided into three main processes. The first stage (≤150 °C) can be attributed to the physically sorbed water possibly with residual solvent on the surface. In the second stage (150–500 °C), the weight losses of the MWCNTs, GONRs, GONRs–DAMN and AOGONRs are 12.0%, 20.4%, 17.8%, 13.4%, respectively. For the GONRs, this is attributed to the decomposition of the oxygen-containing functional groups. For GONRs–DAMN and AOGONRs, the weight loss is owing to pyrolysis of the functional groups (mainly cyano groups and amidoxime groups) covalently bound on the framework of the carbon-based nanomaterials.22,33 The last stage (≥500 °C) is due to the release of CO2 from the burning of carbon. The TGA profiles confirm that a significant amount of the functional groups was exposed on the GONR surface after grafting.
Effect of pH
The pH of aqueous solution is an important parameter for uranium(VI) sorption because it affects the surface charge of the sorbent as well as the speciation of the solute. The effect of pH on uranyl ions sorption by AOGONRs was studied, and the results are shown in Fig. 6. The pH value investigated in this study was not higher than 4.5, because uranyl ions in the designed system would precipitate at higher pH values according to the species distribution for U(VI) hydrolysis.26 | ||
| Fig. 6 Effect of pH on U(VI) sorption on MWCNTs, GONRs and AOGONRs. w = 10 mg, C0 = 0.25 mmol L−1, t = 240 min, T = 298 K, and V = 50 mL. | ||
As can be seen from Fig. 6, the sorption amount of U(VI) on the AOGONRs increases drastically with increasing pH, indicating that the sorption process is clearly pH-dependent. The low sorption at lower pH could be due to the protonation of the oxime and imino groups of amidoxime on the AOGONRs and the competition of H+ with the active sites.34 As the pH values increase, the degree of protonation of the oxime groups will be weakened and the hydroxyl proton in the oxime group would be easily stripped off,14 consequently favoring the sorption of UO22+ on the AOGONRs. Furthermore, the sorption amount of U(VI) on the AOGONRs is higher than that on MWCNTs and GONRs, implying that the amidoximation of GONRs can improve the sorption capacity for U(VI).
Kinetic studies
Experiments were performed to study the effect of the contact time on U(VI) sorption on MWCNTs, GONRs and AOGONRs at different times varying from 2 to 120 min. As shown in Fig. 7, it is evident that the sorption amount of U(VI) on the AOGONRs increased rapidly in the first 10 min, and the sorption equilibrium was reached within 20 min. However, the sorption amount of U(VI) on the MWCNTs and GONRs increased slowly and the sorption equilibrium was attained at 60 min. These observations showed that U(VI) sorption on AOGONRs was mainly through surface complexation in a short reaction time.35 | ||
| Fig. 7 Effect of contact time on the U(VI) sorption on MWCNTs, GONRs and AOGONRs. pH = 4.5, w = 10 mg, C0 = 0.25 mmol L−1, T = 298 K, and V = 50 mL. | ||
Two different kinetic models, i.e. pseudo-first-order and pseudo-second-order were employed to describe the kinetic characteristics of the sorption of U(VI) on the three sorbents. The pseudo-first order kinetic model is expressed as eqn (3):
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (3) |
The pseudo-second order kinetic model is always given as eqn (4):
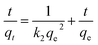 | (4) |
The sorption kinetic parameters in eqn (3) and (4) were calculated from the slopes and intercepts of the plots of ln(qe − qt) versus t and t/qt versus t, and the results are shown in Table 3. Obviously, the highest correlation coefficient value of pseudo-second-order model and the closest qe,cal to qe,exp indicated that the pseudo-second-order model was more suitable to describe the sorption process of U(VI) on MWCNTs, GONRs and AOGONRs. The pseudo-second-order model was based on the assumption that the rate-determining step may be chemisorption.36
| Sorbents | qe (mmol g−1) | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe,cal (mmol g−1) | R2 | k2 (g mmol−1 min−1) | qe,cal (mmol g−1) | R2 | ||
| MWCNTs | 0.051 | 0.0557 | 0.0256 | 0.9716 | 7.41 | 0.052 | 0.9996 |
| GONRs | 0.668 | 0.0582 | 0.3191 | 0.9295 | 0.29 | 0.704 | 0.9987 |
| AOGONRs | 0.941 | 0.2048 | 0.3657 | 0.9388 | 2.02 | 0.948 | 0.9999 |
Sorption isotherms
The sorption isotherms of U(VI) on MWCNTs, GONRs and AOGONRs are presented in Fig. 8. It is obvious that the AOGONRs have a great enhancement in the sorption capacity of U(VI). The maximum sorption amount for U(VI) on the AOGONRs was found to be about 2.112 mmol g−1 (502.6 mg g−1) at 298 K under this system, which is much higher than that of the carbon-based nanomaterials listed in Table 4. The higher U(VI) sorption capacity of AOGONRs could be explained by the chelating groups on the AOGONRs. The presence of nitrogen- and oxygen-containing groups on the AOGONRs surface means that complexes can form with the uranyl species.17,37 AOGONRs with such a high sorption ability towards U(VI) exhibit great potential for applications in the removal and recovery of U(VI) from large volumes of aqueous solutions.| Sorbents | Experimental conditions | Capacity (mg g−1) | Ref. |
|---|---|---|---|
| Pristine CNTs | pH = 5.0, r.t, I = 0.1 M NaClO4 | 4.28 | 32 |
| CNTs treated with HNO3 and H2SO4 | pH = 5.0, r.t, I = 0.1 M NaClO4 | 45.9 | 32 |
| Untreated MWCNTs | pH = 5.0, T = 308 K | 39.5 | 38 |
| Plasma functionalized MWCNTs | pH = 5.6, T = 293 K, I = 0.01 M NaClO4 | 17.35 | 39 |
| Oxidized MWCNTs | pH = 5.0, T = 298 K, I = 0.01 M NaClO4 | 33.32 | 40 |
| MWCNTs grafted with chitosan | pH = 5.0, T = 293 K, I = 0.01 M NaClO4 | 34.55 | 41 |
| MWCNTs grafted with CMC | pH = 5.0, T = 298 K, I = 0.01 M NaClO4 | 111.9 | 28 |
| Graphene oxide nanosheets | pH = 5.0, T = 293 K, I = 0.01 M NaClO4 | 97.5 | 5 |
| Graphene oxide nanosheets | pH = 4.0, r.t | 299 | 7 |
| Reduced graphene oxide nanosheets | pH = 4.0, r.t | 47 | 7 |
| Magnetic graphene/iron oxides | pH = 5.5, T = 293 K, I = 0.01 M NaClO4 | 69.49 | 42 |
| Amidoximated magnetite/graphene oxide composites | pH = 5.0, T = 298 K, I = 0.01 M NaClO4 | 284.9 | 25 |
| Graphene oxide-activated carbon | pH = 5.5, T = 298 K | 298 | 43 |
| Graphene oxide nanosheets | pH = 4.0, T = 303 K, I = 0.01 M NaClO4 | 208.3 | 44 |
| AOGONRs | pH = 4.5, T = 298 K, I = 0.01 M NaClO4 | 502.6 | This work |
Furthermore, experimental data were evaluated by both Langmuir isotherm and Freundlich isotherm models. The Langmuir isotherm model assumes that the sorption occurred on a homogeneous surface by monolayer sorption. It can be expressed as:
 | (5) |
The Freundlich model is usually appropriate to describe heterogeneous systems in the following equation:
| qe = KFC1/ne | (6) |
The relative parameters calculated from the two models are listed in Table 5. The experimental data fit the Langmuir model better than the Freundlich one, suggesting that U(VI) absorbed on the surface to form a monolayer coverage and that chemisorption is the predominant mechanism.
| Sorbents | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mmol g−1) | b (L mmol−1) | R2 | KF [(mmol g−1) (L mmol−1)1/n] | n | R2 | |
| MWCNTs | 0.117 | 3.579 | 0.9795 | 0.0954 | 2.452 | 0.9030 |
| GONRs | 1.917 | 4.307 | 0.9947 | 1.9072 | 2.158 | 0.9337 |
| AOGONRs | 2.353 | 10.465 | 0.9976 | 2.4286 | 3.119 | 0.9343 |
Thermodynamic studies
The effect of temperature on U(VI) sorption on MWCNTs, GONRs and AOGONRs is also given in Fig. 9. The sorption amount of U(VI) increases gradually with an increase in temperature, which suggested that a higher temperature was beneficial to the U(VI) sorption process. | ||
| Fig. 9 Effect of temperature on the U(VI) sorption on MWCNTs, GONRs and AOGONRs, pH = 4.5, w = 10 mg, C0 = 0.25 mmol L−1, t = 240 min and V = 50 mL. | ||
The temperature dependence of a sorption process is associated with changes in several thermodynamic parameters such as the standard free energy (ΔG), enthalpy (ΔH) and entropy (ΔS), which are calculated using the following eqn (7) and (8):
 | (7) |
| ΔG = ΔH − TΔS | (8) |
The values of ΔH and ΔS listed in Table 6 were calculated from the slope and intercept of the plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd versus T−1, and the values of ΔG were obtained using eqn (8). The positive value of ΔH shows the endothermic nature of the sorption process on the three sorbents. The positive value of ΔS suggests an increased randomness at the solid–liquid interface during the sorption on the MWCNTs, GONRs and AOGONRs. The negative value of ΔG indicates that the adsorption reaction is spontaneous. In addition, the values of ΔG for MWCNTs, GONRs and AOGONRs at 298 K were −11.47, −19.73 and −22.10 kJ mol−1, which indicated that the sorption of U(VI) on the AOGONRs was more favorable than that on the MWCNTs and GONRs.
Kd versus T−1, and the values of ΔG were obtained using eqn (8). The positive value of ΔH shows the endothermic nature of the sorption process on the three sorbents. The positive value of ΔS suggests an increased randomness at the solid–liquid interface during the sorption on the MWCNTs, GONRs and AOGONRs. The negative value of ΔG indicates that the adsorption reaction is spontaneous. In addition, the values of ΔG for MWCNTs, GONRs and AOGONRs at 298 K were −11.47, −19.73 and −22.10 kJ mol−1, which indicated that the sorption of U(VI) on the AOGONRs was more favorable than that on the MWCNTs and GONRs.
| Sorbents | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | ΔG (kJ mol−1) | |||
|---|---|---|---|---|---|---|
| 283 (K) | 298 (K) | 313 (K) | 328 (K) | |||
| MWCNTs | 4.82 | 54.69 | −10.65 | −11.47 | −12.30 | −13.12 |
| GONRs | 4.14 | 80.11 | −18.53 | −19.73 | −20.93 | −22.14 |
| AOGONGs | 9.99 | 107.72 | −20.49 | −22.10 | −23.72 | −25.34 |
Effect of ionic strength
The effect of ionic strength on the sorption capacity of AOGONRs for U(VI) at different NaNO3 concentrations (0–5.0 mol L−1) was investigated. As shown in Fig. 10, the influence of U(VI) sorption on AOGONRs is considerably negligible, which further confirmed the higher affinity of the AOGONRs toward U(VI). The characteristics of the as-synthesized sorbent could be favorable for use in certain key steps in any future sustainable nuclear fuel cycle. | ||
| Fig. 10 Effect of ionic strength on the sorption of U(VI) on AOGONRs. pH = 4.5, w = 10 mg, C0 = 0.25 mmol L−1, t = 240 min, T = 298 K and V = 50 mL. | ||
Effect of competitive ions
To evaluate the sorption selectivity of both the GONRs and AOGONRs, the effect of competitive cations was investigated in a simulated nuclear industrial effluent with 12 main sensible nuclides including uranyl ions. As shown in Fig. 11a, it is obviously noticeable that chemical modification has brought about a distinct increase in the U(VI) sorption capacity. The total sorption capacity of the AOGONRs for all cations reached 2.27 mmol g−1, which was much higher than that of the GONRs (1.46 mmol g−1). Meanwhile, the sorption capacity for U(VI) increased from 0.41 mmol g−1 for the GONRs to 1.35 mmol g−1 for the AOGONRs, accounting for about 59.4% of the total sorption amount, which indicated that the AOGONRs had markedly higher affinity towards U(VI) ions. On the other hand, this selectivity can be further clarified by distribution coefficients (Kd). As shown in Fig. 11b, the Kd of AOGONRs reached a high value of up to nearly 6000 mL g−1 for U(VI) and was lower (<500 mL g−1) for other coexistent ions, which suggested that amidoxime-functionalized GONRs exhibited a desirable selectivity for U(VI) ions over a range of competing metal ions. | ||
| Fig. 11 (a) Competitive sorption capacities; (b) the Kd of coexistent ions of U(VI) on GONRs and AOGONRs. C0 = 0.5 mmol L−1 for all cations, pH = 4.5, t = 240 min, w/V = 0.2 g L−1, T = 298 K. | ||
Sorption mechanism
In order to further investigate the interaction mechanism between U(VI) and the AOGONRs at a molecular level, XPS scans of the AOGONRs before and after U(VI) sorption (denoted as AOGONRs–U(VI)) were measured. As shown in Fig. 12a, peaks of C 1s, O 1s and N 1s are seen for the expected components of AOGONRs, and the U 4f level is also detected, suggesting that uranium is adsorbed onto the surface of the AOGONRs. Fig. 12b shows the presence of the characteristic doublets of U 4f5/2 and U 4f7/2 at 393.0 and 382.3 eV with a splitting of about 10.7 eV.23,45 The U 4f spectrum can be resolved into two peaks: the peak at 382.9 eV is assigned to the free uranyl adsorbed on AOGONRs, and the peak at 382.0 eV is attributed to the covalent bond of AO–U(VI).30,46 | ||
| Fig. 12 (a) The typical XPS survey spectra of AOGONRs and AOGONRs–U(VI). High resolution XPS spectra of (b) U 4f, (c) O 1s and (d) N 1s. | ||
The observed spectra of the O 1s and N 1s peaks of AOGONRs and AOGONRs–U(VI) were simulated using two-component Gaussian–Lorentzian sum functions. Fig. 12c shows the O 1s spectra of AOGONRs before and after U(VI) sorption. For the AOGONRs, the peak can be decomposed into three peaks at 531.5, 533.2 and 535.3 eV, which can be assigned to bridging –OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and alcoholic C–O, respectively.30 Compared to the AOGONRs, a higher binding energy of the bridging –OH peak is observed and the relative intensities of the C
O and alcoholic C–O, respectively.30 Compared to the AOGONRs, a higher binding energy of the bridging –OH peak is observed and the relative intensities of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O peaks of AOGONRs–U(VI) decrease. The great variation of the O 1s peak before and after U(VI) sorption indicates that UO22+ can form strong complexes with oxygen-containing functional groups.
O and C–O peaks of AOGONRs–U(VI) decrease. The great variation of the O 1s peak before and after U(VI) sorption indicates that UO22+ can form strong complexes with oxygen-containing functional groups.
As shown in Fig. 12d, the N 1s spectrum in the AOGONR composites could be separated into two peaks: the peak at 399.6 eV corresponds to NH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH, and the peak at 401.5 eV is related to cationic nitrogen atoms (N+).47 Compared to the AOGONRs, the position of NH2–C
NOH, and the peak at 401.5 eV is related to cationic nitrogen atoms (N+).47 Compared to the AOGONRs, the position of NH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH after U(VI) sorption was shifted to a higher binding energy. This change could be ascribed to the formation of the complexes between NH2–C
NOH after U(VI) sorption was shifted to a higher binding energy. This change could be ascribed to the formation of the complexes between NH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH and UO22+, in which UO22+ shares electrons with the nitrogen atom in the amidoxime group.48,49
NOH and UO22+, in which UO22+ shares electrons with the nitrogen atom in the amidoxime group.48,49
Based on analysis of the XPS spectra and previous sorption behavior, the high sorption ability of AOGONRs is largely due to large number of nitrogen- and oxygen-containing functional groups in the amidoxime groups, which can easily form strong complexes with UO22+ on the AOGONRs surface.
Desorption and reusability studies
Repeated availability is also very important for practical application when evaluating the economy and applicability of sorbents. In this work, desorption experiments of U(VI) were performed with HCl solutions in the concentration range from 0.1 to 2.0 mol L−1. As shown in Fig. 13, about 98% of U(VI) ions can be desorbed using 0.5 mol L−1 HCl. Consequently, 0.5 mol L−1 HCl aqueous solution was selected as the desorbing agent for AOGONRs.To assess the reusability of the sorbent, the regenerated AOGONRs were used for five consecutive sorption/desorption cycles. As shown in Fig. 14, the sorption amount of U(VI) decreased slightly from 0.94 mmol g−1 to 0.89 mmol g−1 after five consecutive cycles, indicating that the AOGONRs present excellent reusability and can be used as a good sorbent applied in the field of U(VI) removal and recovery.
 | ||
| Fig. 14 Regenerated use of AOGONRs. pH = 4.5, w = 10 mg, C0 = 0.25 mmol L−1, t = 240 min, and T = 298 K. | ||
Conclusions
An amidoxime-functionalized GONRs sorbent was successfully synthesized in the present work. The raw materials of MWCNTs used to form GONRs are commercially available and cheaper than graphene. AOGONRs have not only a strong affinity, but also demonstrate high selectivity toward uranium(VI) even in a multi-ion system and test solution with weak acidity and high ionic strength. Uranium sorption on the AOGONRs was a pH-dependent, ionic strength-independent, fast, endothermic, spontaneous and a pseudo-second order process. Repeated sorption–desorption experiments indicated that the AOGONRs can be effectively regenerated and reused for U(VI) sorption without an obvious loss in the sorption amount. The results suggested that the new GONRs-based sorbent may be a promising candidate for applications in the selective separation of uranium from nuclear fuel effluents, as well as other related water sources.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants No. 91226108), NASF (Grants No. U1330125), the Ph.D. Programs Foundation of Ministry of Education of China (Grants No. 20110181120001), and the National Fund of China for Fostering Talents in Basic Science (J1210004).References
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed
.
- H. Chang and H. Wu, Energy Environ. Sci., 2013, 6, 3483–3507 CAS
.
- D. K. James and J. M. Tour, Acc. Chem. Res., 2012, 46, 2307–2318 CrossRef PubMed
.
- L. Tan, Y. Wang, Q. Liu, J. Wang, X. Jing, L. Liu, J. Liu and D. Song, Chem. Eng. J., 2015, 259, 752–760 CrossRef CAS PubMed
.
- G. Zhao, T. Wen, X. Yang, S. Yang, J. Liao, J. Hu, D. Shao and X. Wang, Dalton Trans., 2012, 6182–6188 RSC
.
- A. Y. Romanchuk, A. S. Slesarev, S. N. Kalmykov, D. V. Kosynkin and J. M. Tour, Phys. Chem. Chem. Phys., 2013, 15, 2321–2327 RSC
.
- Z. Li, F. Chen, L. Yuan, Y. Liu, Y. Zhao, Z. Chai and W. Shi, Chem. Eng. J., 2012, 210, 539–546 CrossRef CAS PubMed
.
- N. Pan, D. Guan, T. He, R. Wang, I. Wyman, Y. Jin and C. Xia, J. Radioanal. Nucl. Chem., 2013, 298, 1999–2008 CrossRef CAS
.
- Y. Sun, Q. Wang, C. Chen, X. Tan and X. Wang, Energy Environ. Sci., 2012, 46, 6020–6027 CAS
.
- L. Ma, J. Wang and F. Ding, ChemPhysChem, 2013, 14, 47–54 CrossRef CAS PubMed
.
- A. Hirsch, Angew. Chem., Int. Ed., 2009, 48, 6594–6596 CrossRef CAS PubMed
.
- D. V. Kosynkin, A. L. Higginbotham, A. Sinitskii, J. R. Lomeda, A. Dimiev, B. K. Price and J. M. Tour, Nature, 2009, 458, 872–876 CrossRef CAS PubMed
.
- Y. Wang, Z. Wang, Z. Gu, J. Yang, J. Liao, Y. Yang, N. Liu and J. Tang, J. Radioanal. Nucl. Chem., 2015, 304, 1329–1337 CrossRef CAS
.
- Y. Zhao, J. Li, L. Zhao, S. Zhang, Y. Huang, X. Wu and X. Wang, Chem. Eng. J., 2014, 235, 275–283 CrossRef CAS PubMed
.
- C.-Z. Wang, J.-H. Lan, Q.-Y. Wu, Q. Luo, Y.-L. Zhao, X.-K. Wang, Z.-F. Chai and W.-Q. Shi, Inorg. Chem., 2014, 53, 9466–9476 CrossRef CAS PubMed
.
- X. Liu, H. Liu, H. Ma, C. Cao, M. Yu, Z. Wang, B. Deng, M. Wang and J. Li, Ind. Eng. Chem. Res., 2012, 51, 15089–15095 CrossRef CAS
.
- N. Horzum, T. Shahwan, O. Parlak and M. M. Demir, Chem. Eng. J., 2012, 213, 41–49 CrossRef CAS PubMed
.
- Y. Yue, X. Sun, R. T. Mayes, J. Kim, P. F. Fulvio, Z. Qiao, S. Brown, C. Tsouris, Y. Oyola and S. Dai, Sci. China: Chem., 2013, 56, 1510–1515 CrossRef CAS
.
- J. Górka, R. T. Mayes, L. Baggetto, G. M. Veith and S. Dai, J. Mater. Chem. A, 2013, 1, 3016 Search PubMed
.
- M. Carboni, C. W. Abney, K. M. L. Taylor-Pashow, J. L. Vivero-Escoto and W. Lin, Ind. Eng. Chem. Res., 2013, 52, 15187–15197 CrossRef CAS
.
- D. Shao, J. Li and X. Wang, Sci. China: Chem., 2014, 1–10 Search PubMed
.
- X. Yang, J. Li, J. Liu, Y. Tian, B. Li, K. Cao, S. Liu, M. Hou, S. Li and L. Ma, J. Mater. Chem. A, 2014, 2, 1550 CAS
.
- A. Gopalan, M. F. Philips, J.-H. Jeong and K.-P. Lee, J. Nanosci. Nanotechnol., 2014, 14, 2451–2458 CrossRef CAS PubMed
.
- J. Geng, L. Ma, H. Wang, J. Liu, C. Bai, Q. Song, J. Li, M. Hou and S. Li, J. Nanosci. Nanotechnol., 2012, 12, 7354–7363 CrossRef CAS PubMed
.
- Y. Zhao, J. Li, S. Zhang, H. Chen and D. Shao, RSC Adv., 2013, 3, 18952 RSC
.
- Y. Wang, Z. Gu, J. Yang, J. Liao, Y. Yang, N. Liu and J. Tang, Appl. Surf. Sci., 2014, 320, 10–20 CrossRef CAS PubMed
.
- A. L. Higginbotham, D. V. Kosynkin, A. Sinitskii, Z. Sun and J. M. Tour, ACS Nano, 2010, 4, 2059–2069 CrossRef CAS PubMed
.
- D. Shao, Z. Jiang, X. Wang, J. Li and Y. Meng, J. Phys. Chem. B, 2009, 113, 860–864 CrossRef CAS PubMed
.
- J. Wang, Z. Shi, Y. Ge, Y. Wang, J. Fan and J. Yin, J. Mater. Chem., 2012, 22, 17663–17670 RSC
.
- W. Song, X. Wang, Q. Wang, D. Shao and X. Wang, Phys. Chem. Chem. Phys., 2015, 17, 398–406 RSC
.
- Y. Wang, Z. Shi and J. Yin, J. Phys. Chem. C, 2010, 114, 19621–19628 CAS
.
- A. Schierz and H. Zanker, Environ. Pollut., 2009, 157, 1088–1094 CrossRef CAS PubMed
.
- S. M. Badawy, H. H. Sokker, S. H. Othman and A. Hashem, Radiat. Phys. Chem., 2005, 73, 125–130 CrossRef CAS PubMed
.
- K. Singh, C. Shah, C. Dwivedi, M. Kumar and P. N. Bajaj, J. Appl. Polym. Sci., 2013, 127, 410–419 CrossRef CAS PubMed
.
- Y. Sun, C. Ding, W. Cheng and X. Wang, J. Hazard. Mater., 2014, 280, 399–408 CrossRef CAS PubMed
.
- B. Li, L. Ma, Y. Tian, X. Yang, J. Li, C. Bai, X. Yang, S. Zhang, S. Li and Y. Jin, J. Hazard. Mater., 2014, 271, 41–49 CrossRef CAS PubMed
.
- S. P. Dubey, A. D. Dwivedi, M. Sillanpää, Y.-N. Kwon and C. Lee, RSC Adv., 2014, 4, 46114–46121 RSC
.
- I. I. Fasfous and J. N. Dawoud, Appl. Surf. Sci., 2012, 259, 433–440 CrossRef CAS PubMed
.
- M. Song, Q. Wang and Y. Meng, J. Radioanal. Nucl. Chem., 2012, 293, 899–906 CrossRef CAS
.
- Y. Sun, S. Yang, G. Sheng, Z. Guo and X. Wang, J. Environ. Radioact., 2012, 105, 40–47 CrossRef CAS PubMed
.
- D. Shao, J. Hu and X. Wang, Plasma Processes Polym., 2010, 7, 977–985 CrossRef CAS PubMed
.
- P. Zong, S. Wang, Y. Zhao, H. Wang, H. Pan and C. He, Chem. Eng. J., 2013, 220, 45–52 CrossRef CAS PubMed
.
- S. Chen, J. Hong, H. Yang and J. Yang, J. Environ. Radioact., 2013, 126, 253–258 CrossRef CAS PubMed
.
- C. Ding, W. Cheng, Y. Sun and X. Wang, Dalton Trans, 2014, 43, 3888–3896 RSC
.
- D. Shao, G. Hou, J. Li, T. Wen, X. Ren and X. Wang, Chem. Eng. J., 2014, 255, 604–612 CrossRef CAS PubMed
.
- X. Wang, S. Zhang, J. Li, J. Xu and X. Wang, Inorg. Chem. Front., 2014, 1, 641–648 RSC
.
- H. Chen, D. Shao, J. Li, A. Alsaedi and X. Wang, Chem. Eng. J., 2014, 254, 623–634 CrossRef CAS PubMed
.
- W. Song, M. Liu, R. Hu, X. Tan and J. Li, Chem. Eng. J., 2014, 246, 268–276 CrossRef CAS PubMed
.
- Z. Yu, E. Kang and K. Neoh, Langmuir, 2002, 18, 10221–10230 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2015 |