β-Cyclodextrin modified graphene oxide–magnetic nanocomposite for targeted delivery and pH-sensitive release of stereoisomeric anti-cancer drugs†
Abstract
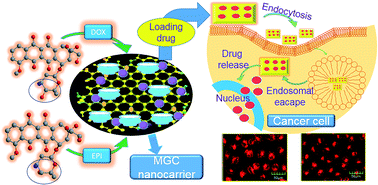
β-Cyclodextrin modified graphene oxide–magnetic (MGC) nanocomposite as an innovative drug carrier was the first to be developed via an effective layer-by-layer-assembly method. Doxorubicin hydrochloride (DOX) and epirubicin hydrochloride (EPI) as model drugs were loaded onto the MGC via π–π stacking, hydrogen bonding and hydrophobic interactions. The MGC exhibits remarkably higher loading capacity for DOX (909.09 mg g−1) and EPI (781.25 mg g−1) than magnetic graphene oxide (MG). The release profiles of the drugs are pH-sensitive which would control the release in the acidic cytoplasm of cancer cells. Furthermore, cellular uptake using fluorescein isothiocyanate (FITC) labeled MGC proves successful internalization of MGC into the cytoplasm of MCF-7 cells. The fluorescence images demonstrate that MGC/DOX, to a certain extent, displays a more excellent delivery and superior release than MGC/EPI, due to the chiral selective function of β-cyclodextrin (β-CD). The pure MGC shows no obvious cytotoxicity while drug loaded MGC reveals significantly high potency for killing MCF-7 breast cancer cells, suggesting that multi-functionalized MGC is an efficient nanoplatform for targeted delivery and controlled release of stereoisomeric anticancer drugs for biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...