PSS-GN nanocomposites as highly-efficient peroxidase mimics and their applications in colorimetric detection of glucose in serum†
Abstract
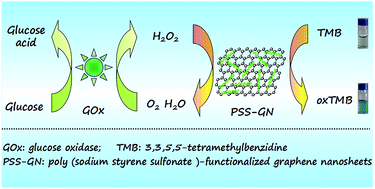
In this report, poly(sodium styrene sulfonate)-functionalized graphene nanosheets (PSS-GN) with good water dispersibility were prepared and for the first time proposed as a peroxidase-like mimic. The intrinsic catalytic activity of PSS-GN nanocomposites was investigated and the results showed that PSS-GN nanocomposites had higher mimetic enzyme catalytic activity compared with horseradish peroxidase (HRP), which might be due to the stronger binding affinity between negatively charged PSS and the positively charged peroxide substrate (3,3,5,5-tetramethylbenzidine, TMB). On the basis of the high catalyze activity, these PSS-GN nanocomposites were utilized for sensitive and selective colorimetric detection of H2O2 as well as glucose. The linearity between analyte concentration and system absorption ranged from 0.005 to 1 mM for H2O2 and 0.006 to 0.4 mM for glucose with a detection limit of 0.15 μM and 0.28 μM, respectively. Moreover, this strategy was further utilized to determine the concentrations of glucose in serum samples with satisfying results. This assay exhibits good analytical performance, acceptable stability and good selectivity. We envision that this sensing system could be used as a tool for a wide range of potential applications in enzyme-based chemical and biological analysis.

 Please wait while we load your content...
Please wait while we load your content...