Effects of spermine on the morphology, digestive enzyme activities, and antioxidant status of jejunum in suckling rats
Abstract
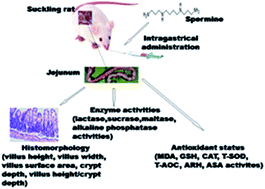
Spermine is a ubiquitous cellular component that plays vital roles in the maintenance of nucleic acids, regulation of kinase activities, protein synthesis, control of ion channel activities and renewal of the gut epithelium. However, knowledge of the effects of spermine supplementation and its duration extension on intestinal growth and antioxidant capacity is lacking. The present work aims to investigate the effects of spermine administration and its extended supplementation on the morphology, digestive enzyme activities, and antioxidant status of the jejunum in suckling rats. The rats received 0.2 μmol g−1 body weight of either spermine or saline solution via intragastric ingestion for 3 or 7 d, and jejunum samples obtained 24 h after the last spermine supplementation were analyzed. The results demonstrated that the specific activity of maltase and the total antioxidant capacity (T-AOC) in rat jejunum were significantly increased by 105.5% and 11.1%, respectively; in contrast, lactase activity was significantly decreased by 34.8% (spermine group versus the control group). Time extension of spermine administration (7 d) significantly increased the villus height, villus width, surface area, and crypt depth in rat jejunum by 11.2%, 18.2%, 5.9%, and 50%, respectively, but significantly decreased the specific activities of lactase, maltase, alkaline phosphatase, and malondialdehyde content by 26.8%, 36.4%, 41.3%, and 26.0% (P < 0.05), respectively. Protein content, sucrase, catalase, T-SOD, and anti-superoxide anion activities were also increased by 23.1%, 424.2%, 45.7%, 11.7%, and 26.4% (P < 0.05), respectively, relative to the levels observed during spermine administration for 3 d. Taken together, the results suggest that spermine administration and extension of its supplementation duration can accelerate gut development and enhance antioxidant properties.

 Please wait while we load your content...
Please wait while we load your content...