Dihydroisoquinoline copper(ii) complexes: crystal structures, cytotoxicity, and action mechanism†
Abstract
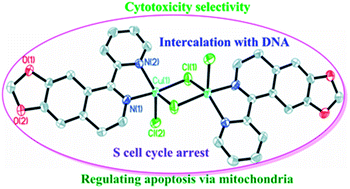
Three new copper(II) complexes of 4,5-methylenedioxy-1-pyridinedihydroisoquinoline (MPDQ), [Cu2(MPDQ)2Cl4] (1), [Cu(MPDQ)(H2O)(SO4)] (2), and [Cu2(MPDQ)2(C2O4)(ClO4)2] (3) were synthesized and characterized. They exhibit enhanced cytotoxicity against the tested human tumor cells BEL-7404, SK-OV-3, HepG2, A549, A375, MGC-803 and NCI-H460 compared to MPDQ and the corresponding copper(II) salts with IC50 values of 1.41–34.54 μM. Complex 1 can induce apoptotic death in BEL-7404 by S cell cycle arrest and complex 1-induced apoptosis, which were involved in an intrinsic pathway, including up-regulating P53 and down-regulating Bcl-2 and mitochondrial membrane potential, leading to sequential activation of caspase-9 and caspase-3. ICP-MS testing implied that the copper(II) complexes could enter cells and DNA was one important target; DNA binding studies revealed that intercalation might be the most probable binding mode of the new Cu(II) complexes with ct-DNA.


 Please wait while we load your content...
Please wait while we load your content...