3D printing of an extremely tough hydrogel
Abstract
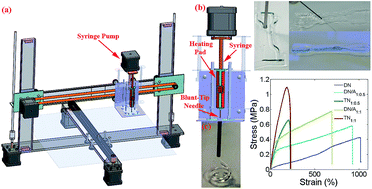
Because of their low viscosity and large gelling temperature range, precise 3D printing of agar hydrogels has not been achieved even though the agar double network hydrogels are tough and self-recoverable. In this work, a super tough agar double network hydrogel was precisely printed by adding alginate. The addition of alginate not only increases the ink viscosity and printable period, but also improves its rheological characteristics towards precise processing control. Moreover, the entanglement of the alginate chains with the agar double network hydrogel restricts the agar helical chain bundles from pulling out under stress, which toughens the hydrogel.

 Please wait while we load your content...
Please wait while we load your content...