Annuloselectivity and stereochemistry in the sulfa-Staudinger cycloadditions of cyclic imines†
Abstract
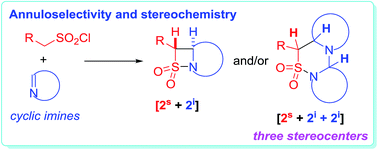
The annuloselectivity and the stereochemistry in the sulfa-Staudinger cycloadditions of cyclic imines are controlled by the ring size of the cyclic imines. Intrinsically, it is the steric hindrance of cyclic imines that controls the annuloselectivity, as well as the stereochemistry in the [2s + 2i + 2i] annulations. A stepwise [4 + 2] annulation mechanism, which incorporates an intermolecular addition, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond isomerization, and subsequently intramolecular addition, is proposed to explain the different stereochemistry in the [2s + 2i + 2i] annulations. The intermolecular addition is regarded as the key stereo-determining step. Firstly, the C3 and C5 stereochemistry is kinetically controlled by the endo or exo addition of imines to the key zwitterionic 2,3-thiaza-1,4-butadiene-type intermediates, and then the C5 and C6 stereochemistry is thermodynamically controlled by the isomerization of the C
S bond isomerization, and subsequently intramolecular addition, is proposed to explain the different stereochemistry in the [2s + 2i + 2i] annulations. The intermolecular addition is regarded as the key stereo-determining step. Firstly, the C3 and C5 stereochemistry is kinetically controlled by the endo or exo addition of imines to the key zwitterionic 2,3-thiaza-1,4-butadiene-type intermediates, and then the C5 and C6 stereochemistry is thermodynamically controlled by the isomerization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond in the zwitterionic endo- or exo-adducts generated from the previous step. The intramolecular addition does not affect the stereochemical outcomes of the [2s + 2i + 2i] annulations.
S bond in the zwitterionic endo- or exo-adducts generated from the previous step. The intramolecular addition does not affect the stereochemical outcomes of the [2s + 2i + 2i] annulations.

 Please wait while we load your content...
Please wait while we load your content...