Spin-coatable, photopatternable magnetic nanocomposite thin films for MEMS device applications
M. Kandpal*a,
C. Sharanb,
V. Palaparthya,
N. Tiwarya,
P. Poddarb and
V. Ramgopal Rao*a
aCentre for Excellence in Nanoelectronics, Department of Electrical Engineering, Indian Institute of Technology, Bombay, 400076, India. E-mail: rrao@ee.iitb.ac.in; mkandpal@iitb.ac.in
bPhysical & Materials Chemistry Division, CSIR-National Chemical Laboratory, Pune, 411008, India
First published on 2nd October 2015
Abstract
Magnetic nanomaterials' (especially metals) air stability and compatibility with standard micro-fabrication technologies are often a concern for development of MEMS-based magnetic devices. In this paper, we report an air-stable, photo-patternable and spin-coatable magnetic thin film preparation process for MEMS applications. This magnetic nanocomposite thin film was prepared by incorporating carbon capped ferromagnetic cobalt nanoparticles of dimension 20–80 nm into the SU-8 matrix. TEM, XRD and EDAX analyses were done, to investigate the crystal structure, dispersion and phase stability of the films. The SQUID magnetometry and MFM measurements of the film confirmed its magnetic response at room temperature and the retention of its magnetic properties over a period of time. The material compatibility for MEMS device applications was demonstrated through fabrication of a suspended circular membrane of radius ∼250 μm, having four U-shaped beams, of dimension ∼270 × 50 μm each. Three conventional lithography steps and a sacrificial release layer of ∼1 μm thick oxide was used for the fabrication. The membrane was characterized by evaluating its spring constant and resonant frequency. The spring constant and resonant frequencies were estimated to be ∼4.2 N m−1 and ∼29 kHz respectively. Finally, we demonstrated the actuation of the magnetic membrane by an off-chip generated magnetic field, for its possible use as a MEMS device.
1. Introduction
Nanocomposite materials with their well-defined physical and chemical properties have attracted considerable attention in the recent past, for applications in micro-fabrication technologies. The challenge, however, has been the tailoring of the materials' piezoresistive, piezoelectric, magnetic, and chemical properties with high biocompatibility.1–5 Magnetic nanocomposites with different magnetic fillers and a combination of various polymer matrices are areas of current research interest because of their numerous applications in the area of nanoelectronics and biomedical devices.6–11 The biggest challenge with using magnetic materials for device applications arises from their traditional method of deposition; etch chemistry and high affinity towards oxygen. The formation of an oxide layer over a core magnetic material is always undesirable, as it deteriorates the magnetic properties over a period of time.12–14 Therefore, there is a need to develop new types of magnetic materials, which are amenable for micro-fabrication applications. Because of the recent progress in the synthesis of magnetic-capped nanoparticles and significant innovations in nanocomposite thin films, there is an opportunity to develop low cost photopatternable magnetic nanocomposite materials. During the last decade, a significant progress has been made in the development of various magnetic nanoparticle synthesis for applications in novel imaging, specific drug delivery and recovery of catalysts. Previously, polymer based magnetic nanocomposite materials have been explored for electromagnetic shielding applications by various research groups.15–17 Also, in the past researchers have explored these materials for their application in micro robotics and energy scavenging. Ergeneman et al. uses nickel–cobalt as a material for realization of micro resonator which utilizes the magnetic actuation and readout system for detection of vibration.18 Sutter et al. also demonstrated a cantilever based MEMS actuator, by incorporation of the super paramagnetic Fe3O4 nanoparticles into SU-8 matrix.19,20 Recently, Alfadhel et al. reported a magnetic flow sensor, which was realized with integration of iron nanowires into polydimethylsiloxane (PDMS).21 Similarly, Singh et al. also reported a cobalt based PDMS nanocomposite for actuation applications.22 PDMS based nanocomposites have an issue with patterning, therefore, it is tough to pattern them using a standard micro fabrication technology. In this work, we report development of air stable, low cost, spin-coatable, magnetic nanocomposite, which can be used for fabrication of MEMS structures. For this work, carbon coated cobalt nanoparticles were selected as nano fillers, for realization of a photo-patternable magnetic nanocomposite (MNC) thin film, because of their good magnetic properties. SU-8 (ref. 23) was used as matrix for this work, as the integration of these thin films for device applications was the main objective.In the present study, cobalt nano fillers and nanocomposite thin films were characterized by different methods such as X-ray diffraction (XRD), transmission electron microscopy (TEM) and superconducting quantum interference devices (SQUID) with the aim to investigate the morphology, size, phase, dispersion and magnetization. Thereafter, a MEMS device was demonstrated with the fabrication of a magnetic micro membrane. Dynamic characterization of the fabricated magnetic membrane was carried out using a Laser Doppler Vibrometer. In order to study the stiffness of fabricated membrane, a static evaluation of membrane was done with the help of a calibrated nanoindentaion technique. Finally, the magnetic response of the fabricated membrane was analyzed qualitatively using an off-chip generated magnetic field.
2. Result and discussion
2.1. Materials & methods for the synthesis of magnetic nanocomposite (MNC)
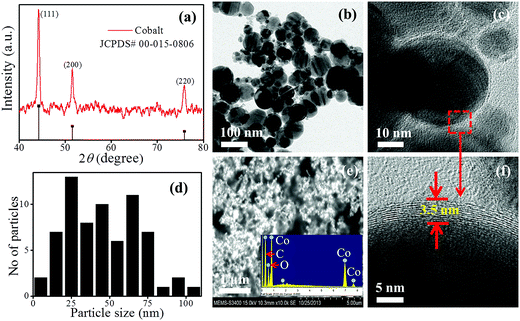
The SU-8/cobalt nanocomposite was prepared by incorporating carbon coated cobalt nanoparticles of size ∼50 nm (Sigma-Aldrich) into a photosensitive polymer SU-8 (Microchem). The illustrations of the used process are as shown in Fig. 1(a and b). Briefly, 25 mg of cobalt nanoparticles were mixed into 1 ml of cyclopentanone (SU-8 Thinner, Microchem). The prepared solution was sonicated for 6 min for proper dispersion of nanoparticles into cyclopentonone. Then a solution of 1 ml SU-8-2002 was added and the mixer was again sonicated for 20 min with an interval of 1 min. In order to compensate the excessive heat generation during sonication process, the whole set was kept in an ice bath. After that, the nanocomposite suspension was transferred over 2′′ silicon wafer using the spin coating process. The spin speed of 2200 rpm for 30 s was used and thickness of 1.5 μm was achieved for MNC film. Then, the spun MNC films were prebaked at 70 °C for 3 min and 90 °C for 4 min for evaporation of the solvent. For patterning these thin films, UV exposure of dose 200 mJ cm−2 was used in two cycles with a 1 min gap time using standard Karl-Suss MJB4 mask aligner. Subsequently, the films were cross-linked by a post-baking process using similar parameters as used for the pre-baking process.2.2. Material characterizations for MNC thin films
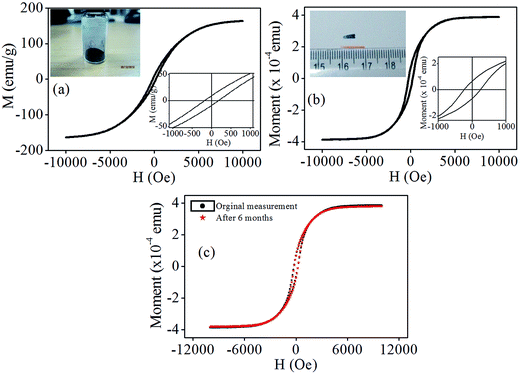
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe applied magnetic field. The inset of figure shows magnified view of M–H curve, with coercivity and remnant magnetization of ∼200 Oe and ∼4 emu g−1 respectively. Fig. 4(b) shows the M–H curve for nanocomposite film and the magnetic moment was found to be 3.9 × 10−4 emu at 10
000 Oe applied magnetic field. The inset of figure shows magnified view of M–H curve, with coercivity and remnant magnetization of ∼200 Oe and ∼4 emu g−1 respectively. Fig. 4(b) shows the M–H curve for nanocomposite film and the magnetic moment was found to be 3.9 × 10−4 emu at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe magnetic fields. The coercivity and remnant magnetic moment was found to be 237 Oe and 6.7 × 10−4 emu respectively. The performance of the device depends on the stability of its magnetic properties over a period of time. The use of carbon coating over the cobalt nanoparticles helps to maintain the magnetic properties, by protecting the cobalt nanoparticles from oxidation, which is responsible for the stability observed in this work. Further, to investigate the magnetic stability of nanocomposite thin film, in ambient condition, the measurement was repeated after ∼180 days and comparison graphs are as shown in Fig. 4(c). This measurement confirms that, the magnetic film retains its magnetic properties over the long span of time, because of the carbon capping of the nanoparticles.
000 Oe magnetic fields. The coercivity and remnant magnetic moment was found to be 237 Oe and 6.7 × 10−4 emu respectively. The performance of the device depends on the stability of its magnetic properties over a period of time. The use of carbon coating over the cobalt nanoparticles helps to maintain the magnetic properties, by protecting the cobalt nanoparticles from oxidation, which is responsible for the stability observed in this work. Further, to investigate the magnetic stability of nanocomposite thin film, in ambient condition, the measurement was repeated after ∼180 days and comparison graphs are as shown in Fig. 4(c). This measurement confirms that, the magnetic film retains its magnetic properties over the long span of time, because of the carbon capping of the nanoparticles.
The MFM measurements taken at different tip-surface distance are as shown in Fig. 5. No significant change occurred in the topography information as seen from the height images (a, c and e). Whereas, their corresponding magnetic phase images (b, d and e) showed significant contrast in topography of surface with respect to the decrease in the cantilever tip height from the surface. Further, to explore the magnetic gradient force on the nanoparticles, we have analyzed the magnitude of the phase changes by studying the line profile taken at different tip-surface distance, as shown in figure (g and h). The maximum phase shift was observed when the tip-surface distance was 30 nm, leading to the increased magnetic interaction between the magnetic probe and nanocomposite surface and depicting an on/off behavior of embedded cobalt nanoparticles inside the SU8 matrix. It can be seen from the MFM images (Fig. 5) that the nanoparticles morphology was different, as compared to the morphology observed in TEM images (Fig. 3(b)). This difference in the images is due to the agglomeration of nanoparticles inside SU-8 matrix and stray magnetization field28 of MFM tip.
3. Fabrication of a micro membrane
A flip chip technique and three levels of lithography process were used for fabrication of a test membrane structure. The schematic representation of process flow is shown in Fig. 6.Briefly, it starts with the RCA cleaned29 p-type silicon wafer of resistivity 4–7 ohm cm, followed by a 1 μm sacrificial oxide layer grown over it. A thin layer of thickness 1.8 μm of SU-8 2002, at a spin speed of 3000 rpm was spun over the oxide layer. This layer was patterned to define the membrane structure. Thereafter, a nanocomposite suspension of 25 mg ml−1 Co concentration was prepared as discussed previously and spin coated over the patterned SU-8 layer. Spin speed of 2200 rpm, prebake temperature of 70 °C for 3 minute and 90 °C for 4 minute were used for evaporation of solvent. Subsequently, a nanocomposite film was exposed to 200 mJ cm−2 in two cycles, with 1 minute interval. For proper crosslinking of exposed films a post-bake process was carried out by setting a similar temperature as that of the pre-bake process. Then an anchor of thickness 250 μm was defined and patterned with a thick SU8-100 resist layer. Structures were released from oxide surface by complete etching of oxide layer using buffer HF concentration of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
4. Device characterizations
Resonant frequency & stiffness measurements for the freely suspended micro membranes were carried out to study the nanomechanical behavior of the fabricated magnetic MEMS devices under dynamic and static conditions.4.1. Static analysis for the evaluation of spring constant
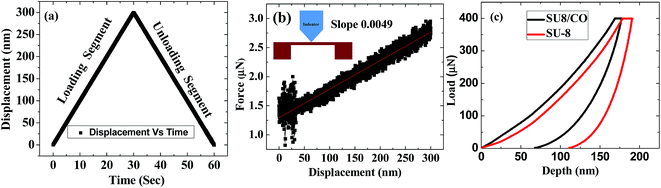
The microscopic image of fabricated membrane is shown in Fig. 6(f). It consists of a membrane which has a circular shape of radius 250 μm supported by four U-shaped cantilever beams of dimension 270 × 50 μm. Beam bending approach using AFM and nanoindentation study are common techniques used for measuring the stiffness of MEMS structures.30 In order to estimate the spring constant of the fabricated MEMS structures, a static analysis was performed using the nanoindentation study. For our study, a displacement controlled-mode was chosen for the analysis. The tip of the nanoindentation apex was placed at the center of the membrane as shown in the inset of Fig. 7(b) so that the load can be uniformly distributed over the beams. A calibrated nanoindentation tip was used for experiments. A constant load of 1.5 μN at a loading rate 10 nm s−1 was used for nanoindentation experiments. Thereafter, the displacement of tip was varied from 0 nm to 300 nm and data for load vs. displacement was analyzed. Membranes showed a linear response on application of a load. Loading and unloading characteristics are as shown in Fig. 7(a). The spring constant of 4.9 N m−1 was estimated from the slope of force–displacement curve as shown in Fig. 7(b). The effect of cobalt nanoparticles on mechanical properties of SU-8 were studied and compared and the results are as shown in Fig. 7(c). These results illustrate that the penetration depth shifted to the lower value of 69 nm, as compared to the earlier value of 139 nm for 400 μN load. Also, Young's modulus of SU-8 increased from 7 to 12 GPa, which is due to higher material density of cobalt nanoparticles.4.2. Resonant frequency measurement
Resonant frequency measurement for freely suspended micro membranes was estimated under dynamic condition of vibration, using a calibrated piezoactuator. A Laser Doppler Vibrometer (LDV) was used for observation of in-plane motion of membrane.31 The membrane's dynamic motion characteristic was analyzed in the range 0–100 kHz. The laser beam of LDV was scanned over the defined grid points on the surface of the membrane and ‘Doppler shift’ of reflected laser beam due to the motion of the membrane was recorded. The recorded mode shapes for the membrane are as shown in Fig. 8(a–c). Three mode shapes for actuation of magnetic membrane were recorded. The mode shape corresponding to 29 kHz (Fig. 8(a)) was identified as its fundamental mode of resonance. In this case, the motion of the suspended membrane was confined to Z direction only which is the desired mode of operation. The other two modes as shown in Fig. 8(b and c) were observed to have the frequencies of 35 and 50 kHz respectively. These mode shapes correspond to torsion motion of membrane. | ||
| Fig. 8 (a) Resonant frequency of fabricated membrane and (b & c), represent the torsion mode actuation characteristic of membrane. | ||
One of the possible applications for this membrane is its use as self-calibrated active mass sensors, so the quality factor needs to be optimized. The quality factor was estimated experimentally from the resonant frequency measurements using the half power point method.32 It can be estimated using the following equation.33
 | (1) |
Where, f0 and Δf are the resonance frequency and bandwidth respectively. As shown in Fig. 8(a), the bandwidth at the midgap was observed to vary from 29.04 kHz to 30.27 kHz. Using the calculation using eqn (1), a quality factor of 23 was observed for the desired fundamental mode.
4.3. Qualitative analysis of magnetic actuation
Sensitivity and accuracy of the fabricated magnetic sensors, depends on the precise control of the deflection of sensors, which is generally in the range of a few nm to a few μm. The micro actuation analysis was done qualitatively with white light interferometry technique.34 The line profiles for membrane surface at various fixed points was first analyzed without the application of magnetic field and thereafter the same pattern was analyzed with the application of magnetic field of 8.7 × 103 A m−1 in Z-direction. For these measurements the vibration was avoided, to exclude the other disturbances. The whole analysis was restricted to a single line, located at 420 μm at X-axis. The recorded topography images are as shown in insets of Fig. 9(a and b) where a significant change in the surface topography was observed, with the change in color contrast of images. The line profile for membrane located at 420 μm was plotted as shown in Fig. 9. | ||
| Fig. 9 Line profiles of membrane with (red) and without (black) the magnetic field. Inset picture (a and b) shows the topography images of the membrane. | ||
A height variation of 10 μm was observed, in the line profiles of membrane about Z-axis on the application of magnetic field. This change is due to the magnetic nature of the embedded Co nanoparticles. When the magnetic field is switched on, a gradient force on the embedded magnetic nanoparticles gets exerted, which changes the position of membrane and hence a change in the line profiles is observed.
5. Conclusions
In summary, a spin coatable, photopatternable nanocomposite with magnetic property was developed for MEMS based magnetic structures. To ensure stability in ambient conditions and better magnetic properties, carbon capped ferromagnetic particles were chosen as fillers for SU-8 based nanocomposite. Thin films of the nanocomposite were evaluated for their phase, elemental analysis and magnetic properties with XRD, TEM, EDAX and MFM techniques. The magnetic nanocomposite thin film showed good magnetic properties and better stability in ambient conditions. Further, for a demonstration of these nanocomposite thin films for MEMS applications, a membrane test structure was fabricated using a flip-chip micro-fabrication technique. Dynamic and static behavior of the fabricated MEMS structures were also studied for their practical applications. A resonant peak at 29 kHz was observed as a fundamental mode of operation. The static evaluation of fabricated membranes showed a spring constant of magnitude 4.3 N m−1. Finally, the fabricated micro membrane was actuated successfully by off-chip generated external magnetic field for its magnetic actuation applications.6. Experimental details
6.1. XRD, SEM, EDAX and TEM measurements
The phase investigation of cobalt nanoparticles was performed with X-ray diffraction (XRD) techniques using Panalytical X'Pert Pro X-ray diffractometer with the copper Kα radiation (λ = 1.5418 Å). The scanning range was set to 10–80° for recording the XRD diffraction patterns. Morphology, dispersion and elemental analysis of cobalt nanoparticles and nanocomposite thin films were investigated by using scanning electron microscopy (model-S-3400N) and transmission electron microscopy, PHILIPS (model no-CM200).6.2. SQUID measurements
The room temperature field dependent magnetic measurements (M vs. H) were carried out on nanoparticles, thin films and devices after fabrication, by tightly fixing them in the sample holder and varying the magnetic field. Vibrating sample magnetometer (LakeShore, VSM – 7410) was used for these experiments.6.3. Nanoindentaion and laser Doppler vibrometer experiments
Laser Doppler instrument, Polytec and nanoindentation Hysitron Inc Minneapolis USA (model TI-900) was used for estimation of spring constant and dynamic analysis of fabricated magnetic membrane.Acknowledgements
The authors wish to acknowledge the support provided by the Department of Electronics and Information Technology, MCIT and DST, Government of India for their financial support and SAIF for providing the SEM and TEM facility. We also extend our acknowledgements to the institute nanoindentation facility, Suman Mashruwala Advanced Micro Engineering Laboratory for the Laser Doppler Vibrometer characterization facility and the IIT Bombay Nano-fabrication facility for the fabrication of MEMS devices.References
- V. Seena, A. Fernandes, P. Pant, S. Mukherji and V. R. Rao, Nanotechnology, 2011, 22, 295501 CrossRef CAS PubMed.
- H. C. Chiamori, J. W. Brwon, E. V. Adhiprakasha, E. T. Hantsoo, J. B. Straalsund, N. A. Melosh and B. L. Pruitt, Microelectron. J., 2008, 39, 228 CrossRef CAS PubMed.
- M. Nordström, S. Keller, M. Lillemose, A. Johansson, S. Dohn, D. Haefliger, G. Blagoi, M. H. Jakobsen and A. Boisen, Sensors, 2008, 8, 1595–1612 CrossRef PubMed.
- K. Prashanthi, M. Naresh, V. Seena, T. Thundat and V. R. Rao, J. Microelectromech. Syst., 2012, 21, 259–261 CrossRef CAS.
- M. Kandpal, C. Sharan, P. Poddar, K. Prashanthi, P. R. Apte and V. R. Rao, Appl. Phys. Lett., 2012, 101, 104102–104105 CrossRef PubMed.
- A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed. Engl., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- J. Gallo, N. J. Long and E. O. Aboagye, Chem. Soc. Rev., 2013, 42, 7816 RSC.
- J. Liu, S. Z. Qiao, Q. H. Hu and G. Q. Lu, Small, 2011, 7, 425–443 CrossRef CAS PubMed.
- S. Behrens, Nanoscale, 2011, 3, 877–892 RSC.
- T. Wen and K. M. Krishnan, J. Phys. D: Appl. Phys., 2011, 44, 393001 CrossRef.
- L. Y-Wei, Z. Q. Feng and L. R. Wei, Chin. Phys. B, 2013, 22(12), 127502 CrossRef PubMed.
- Y. Xu, M. Mahmood, Z. Li, E. Dervishi, S. Trigwell, V. P. Zharov, N. Ali, V. Saini, A. R. Biris, D. Lupu, D. Boldor and A. S. Biris, Nanotechnology, 2008, 19, 435102 CrossRef PubMed.
- H. Li, N. Zhao, C. He, C. Shi, X. Du and J. Li, J. Alloys Compd., 2008, 458, 130–133 CrossRef CAS PubMed.
- N. A. M. Barakat, B. Kim, S. J. Park, Y. Jo, M. H. Jung and H. Y. Kim, J. Mater. Chem., 2009, 19, 7371–7378 RSC.
- J. Gass, P. Poddar, J. Almand, S. Srinath and H. Srikanth, Adv. Funct. Mater., 2006, 16, 71–75 CrossRef CAS PubMed.
- P. Poddar, J. L. Wilson, H. Srikanth, S. A. Morrison and E. E. Carpenter, Nanotechnology, 2004, 15, 570–574 CrossRef.
- O. Yavuz, M. K. Ram, M. Aldissi, P. Poddar and H. Srikanth, Synth. Met., 2005, 151, 211–217 CrossRef CAS PubMed.
- O. Ergeneman, P. Eberle, M. Suter, G. Chatzipirpiridis, K. M. Sivaraman, S. Pané, C. Hierold and B. J. Nelson, Sens. Actuators, A, 2012, 188, 120–126 CrossRef CAS PubMed.
- M. Sutter, O. Ergeneman, J. Zurcher, C. Moitzi, S. Pane, T. Rudin, S. E. Pratsinis, B. J. Nelson and C. Hierold, Sens. Actuators, B, 2011, 156, 433 CrossRef PubMed.
- M. Sutter, O. Ergeneman, J. Zurcher, S. Schimd, A. Camenzind, B. J. Nelson and C. Hierold, J. Micromech. Microeng., 2011, 21, 025023 CrossRef.
- A. Alfadhel, B. Li, A. Zaher, O. Yassine and J. Kosel, Lab Chip, 2014, 14, 4362–4369 RSC.
- A. Singh, L. Hirsinger, P. Delobelle and C. K. Malek, Microsyst. Technol., 2014, 20, 427–436 CrossRef CAS.
- W. H. Teh, U. Durig, U. Drechsler, C. G. Smith and H. J. Guntherodt, J. Appl. Phys., 2005, 97, 054907 CrossRef PubMed.
- K. Yang, M. Gu, Y. Guo, X. Pan and G. Mu, Carbon, 2009, 47, 1723–1737 CrossRef CAS PubMed.
- A. G. Kahrizsangi, J. Neshati, H. Shariatpanahi and E. Akbarinezhad, Polym. Bull., 2015, 72, 2297–2310 CrossRef.
- M. Vinchurkar, A. Joshi, S. Pandey and V. R. Rao, J. Microelectromech. Syst., 2015, 24, 1111–1116 CrossRef.
- J. A. Sidles, J. L. Garbini, K. J. Bruland, D. Rugar, O. Zuger, S. Hoen and C. S. Yannoni, Rev. Mod. Phys., 1995, 67, 249 CrossRef CAS.
- M. Kleiber, F. Kummerlen, M. Lohndorf, A. Wadas, D. Weiss and R. Wiesendanger, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 5563–5567 CrossRef CAS.
- K. Werner, J. Electrochem. Soc., 1990, 137(6), 1887–1892 CrossRef PubMed.
- E. Finot, A. Passian and T. Thundat, Sensors, 2008, 8, 3497–3541 CrossRef PubMed.
- P. Castellini, B. Marchetti and E. P. Tomasini, Proc. IMAC-XXII, Conf., Exposit. Struct.,Dyn., 2004, pp. 85–94 Search PubMed.
- C. F. Beards, Structural vibration analysis, Wiley, New York, 1983, vol. 74 Search PubMed.
- L. Kiesewetter, J. M. Zhang, D. Houdeau and A. Steckenborn, Sens. Actuators, A, 1992, 35, 153–159 CrossRef.
- J. C. Wyant, White light interferometry, Aero Sense International Society for Optics and Photonics, 2002 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |