From supramolecular hydrogels to functional aerogels: a facile strategy to fabricate Fe3O4/N-doped graphene composites†
Ting Hea,
Zhengyuan Lia,
Zhifang Suna,
Shuzhen Chena,
Rujuan Shenb,
Lunzhao Yic,
Liu Deng*a,
Minghui Yanga,
Hongtao Liua and
Yi Zhang*a
aCollege of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China. E-mail: yzhangcsu@csu.edu.cn; dengliu@csu.edu.cn
bState Key Laboratory of Power Metallurgy, Central South University, Changsha 410083, China
cYunnan Food Safety Research Institute, Kunming University of Science and Technology, Kunming, 650500, China
First published on 7th September 2015
Abstract
This manuscript introduces a simple method to fabricate hybrid aerogels with Fe3O4 nanocrystals/nitrogen-doped graphene (Fe3O4/N-GAs) through one-shot self-mineralization of ferrocenoyl phenylalanine/graphene oxide (Fc-F/GO) supramolecular hydrogels. We found that GO could trigger a sol–gel transition of Fc-F gelators below the critical gelation concentration and the electron microscopic results revealed that the self-assembled Fc-F fibrils tightly bound onto graphene sheets. Upon hydrothermal reaction, Fc moieties in these fibrils could be locally oxidized to Fe3O4 nanocrystals by GO, remaining on the top of reduced GO (RGO) sheets and therefore inhibiting the self-aggregation of graphene nanosheets. After drying, the remains of the supramolecular hybrid hydrogels are presented as the three-dimensional (3D) framework of ultra-thin graphene sheets on which Fe3O4 nanoparticles (NPs) are uniformly immobilized as single crystals. Since the new born Fe3O4 nanocrystals are closely anchored on the graphene sheets, the as-prepared Fe3O4/N-GAs complex shows excellent electrocatalytic activity for the oxygen reduction reaction (ORR, compared to commercial Pt/C). Notably, the Fc-F/GO supramolecular hydrogels act as multifunctional reagents, such as capping agents for preventing graphene nanosheets from stacking and Fe and N sources for Fe3O4/N-GAs. We expect that this intriguing strategy can provide a useful archetypical example in designing nonprecious metal oxides/carbon hybrid materials to serve as substitutes for noble metal catalysts.
Introduction
The development of highly efficient ORR catalysts has been widely considered as a key issue in energy conversion technologies.1–6 Traditional ORR electrocatalysts usually contain precious metals like platinum, gold, palladium, etc. However, the prohibitive cost and scarce supply of these precious metal catalysts limit their large-scale application.7–10 Therefore, alternative ORR catalysts with high catalytic activity but low cost are highly desired. The heteroatom-doped carbon materials have been regarded as the promising type of ORR catalysts. Among them, the N-doped graphene materials have been considered as the hottest topic for investigating heteroatom-doped carbon materials as ORR catalysts.11–15 Comparing to the porous N-graphene materials which is constructed by randomly stacked N-graphene sheets, the N-GAs possess much higher electronic conductivity as a result of the continuous graphene sheets in the 3D networks, facilitating much faster charge transport across graphene sheet junctions.16–18From another point of view, non-precious metals, metal oxides, metal chalcogenides and metal-N4 structured macrocyclic compounds have also been applied to improve ORR catalytic activity.19–24 The transition metal oxides, such as Fe2O3, Fe3O4 and Co3O4 have been convinced as important categories.25–29 It is a pity that these metal oxides frequently suffer from dissolution, sintering and agglomeration, which would induce the catalyst degradation. Besides, the insulating property of the metal oxide further limits electrochemical reaction kinetics. Hence, various strategies have been developed to improve their structural integrity and electronic conductivity. Dai's group reported that Co3O4/graphene hybrids can be prepared through a general two-step method. In the first step, Co3O4 NPs were grown on GO sheets in the presence of NH4OH. Subsequent hydrothermal reaction induced the crystallization of Co3O4 and reduction of GO to graphene.28 The ZnO-coated 3D graphene aerogel templates have been also proposed to prepare Fe3O4 NPs/graphene aerogels via a precipitation reaction in which Fe3O4 NPs replaced ZnO NPs.18 However, despite these efforts, most of metal oxides/graphene nanocomposites exhibit lower activity and poorer conductivity than Pt-based materials.
The combination of transition metal oxides and N-GAs has been demonstrated to be a prospective approach. Such hybrid systems permit the use of advanced properties of both materials, such as high activity of transition metal oxides, rich porosity, 3D conductive networks and excellent mechanical properties of porous graphene films. Recently, a number of smart protocols have been successfully utilized to prepare Fe3O4/N-GAs with perfectly chemical performances. Müllen's group have described the assembly of Fe3O4 NPs in 3D N-GAs via hydrothermally treating the mixture of iron acetate, polypyrrole and GO solution at 180 °C, and then the hybrid system was dehydrated via a freeze-drying process and heated at 600 °C for Fe3O4 NPs crystallization.26 The polypyrrole was introduced as a capping agent for both doping N and preventing graphene nanosheets from stacking.30 This work demonstrates that N-GA is indeed a promising scaffold to improve the catalytic activity and durability of metal oxide NPs. So far, the traditional synthetic strategies for the metal oxides/N-doped carbon materials are generally involving two different ingredients to provide the metal oxides and N sources separately, resulting poor physical contact between the two active substances.16,31–33 In contrast, an in situ fabricating method of metal oxides/N-doped carbon nanomaterials derived from one precursor is highly desired to reduce the space barrier of electron transport.
This paper introduces a convenient fabricating route for preparing Fe3O4/N-doped graphene aerogels directly from supramolecular hybrid hydrogels. The as-prepared Fe3O4 NPs/N-GAs exhibit remarkable catalytic activity toward ORR. As far as we know, there is no report regarding supramolecular hydrogelators for the controllable self-assembly of metal oxide NPs and the doping N on graphene sheets. A single hydrogelator, Fc-F serves as a multifunctional reagent, not only for the Fe and N sources of both Fe3O4 NPs and N-doping graphenes, but also the dispersing agent for preventing graphene nanosheets from stacking. Therefore, the synergistic effect can be optimized by the close location of in situ formed N-graphenes and Fe3O4 NPs catalytic sites.34–38 More significantly, the locally formation of metal oxides/N-GAs derived from supramolecular hydrogels offers a new route to produce the morphology-controlled inorganic NPs in hybrid graphene nanomaterials.
Results and discussion
Synthesis and characterization of supramolecular hydrogels
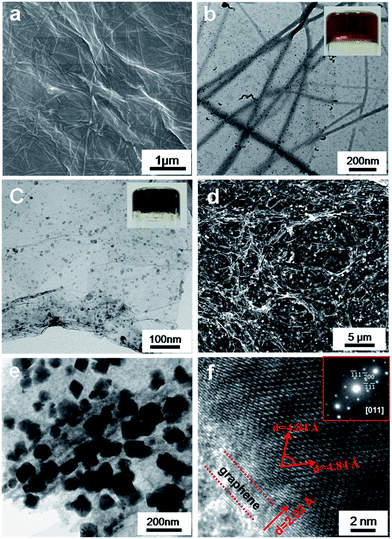
In this paper, we chose a hydrogelator (Fc-F) to implement our proposal, since ferrocene and phenylalanine could be able to act as Fe and N precursors, respectively.39–42 The procedure for preparing the hybrid aerogel is briefly illustrated in Fig. 1.Initially, Fc-F/GO hydrogels were obtained by mixing the Fc-F with GO aqueous suspension (2 mg mL−1 Fc-F and 1 mg mL−1 GO, Fig. 1a and b). After hydrothermal assembly, the supramolecular hybrid hydrogels were subjected to freeze-drying and further thermal treatment to fabricate the hybrid aerogels (Fig. 1c and d). The structures and morphologies of hybrid Fc-F/GO hydrogels were investigated via the scanning electron microscopy (SEM), transmission electron microscopy (TEM) and atomic force microscopy (AFM). Fig. 2a and b and S1† present the microscopic images of Fc-F spheres (2 mg mL−1), GO sheets (1 mg mL−1) and pure Fc-F hydrogels, respectively. Fc-F spheres can aggregate into Fc-F fibrils after the addition of GO. As shown in Fig. 3a and b, the diameter of these Fc-F fibrils in the hybrid system is around 50–100 nm, while the length of these fibrils reaches 1 mm (or longer). Apparently, the Fc-F fibrils and GO nanosheets interlaced with each other in the hybrid hydrogel system. On the one hand, the required concentration of Fc-F applied in forming Fc-F/GO hybrid hydrogels can be significantly lower than the critical gelation concentration with respect to pure Fc-F hydrogels (3.0 mg mL−1), indicating that GO serves as a positive co-gelator besides a solid matrix. On the other hand, the mixture containing enough GO but much less Fc-F (less than 2.0 mg mL−1) cannot form stable hybrid hydrogels, so the Fc-F plays a critical role in facilitating the formation of the mixed hydrogels. Circular dichroism (CD) spectrum was also used to characterize the conformational information of Fc-F/GO hybrid hydrogels.43,44 As shown in Fig. 2c, the hybrid Fc-F/GO hydrogels obviously inherited the chirality derived from Fc-F hydrogels.39–42,45 It is notable that for the hybrid hydrogels, the intensity of the first peak (around 240 nm) decreased while the second peak (around 270 nm) increased comparing with that for the pure Fc-F sol, implying the helix conformation of Fc-F fibrils. Additionally, we explored the viscoelastic properties of the hybrid hydrogels and native hydrogels (CFc-F = 5 mg mL−1) by measuring the storage modulus G′ and the loss modulus G′′. As displayed in Fig. 2d, it can be seen that the viscoelastic properties of the two hydrogels are quite similar and the G′ is about six times higher than G′′, indicating middle-strength hydrogels.42
Fabrication and characterization of Fe3O4/N-GAs
After being hydrothermally incubated at 90 °C for 30 minutes, the color of Fc-F/GO hydrogels changed from brown to black, suggesting the reduction reaction of GO to graphene. On the basis of previous reports, GO exhibits strong oxidative ability to Fe(II), so the Fc moiety in Fc-F fibrils could be oxidized to Fe3O4 NPs.46,47 Moreover, Fc-F fibrils could also act as a dispersant to prevent stacking of the graphene in the hydrothermal reduction process by weakening the bonding interaction between graphene sheets. In this way, Fe3O4 NPs could nucleate and grow on the graphene surface with simultaneous reduction of GO. The growth of Fe3O4 nanocrystals on graphene sheets was confirmed by TEM images. It is notable that Fc-F fibrils disappear, while an interconnected graphene framework co-existing with the small mineralized NPs (with sizes of 10–20 nm) is observed, confirming the effective coupling between the NPs and the graphene sheets (Fig. 3c). However, this well-structured nanomaterials exhibit poor catalytic ability to ORR (results are not shown). Thus for purpose of enhancing the composites catalytic activity, a higher temperature incubation process (heated at 600 °C for 3 h under N2 protection) was performed. Our experimental results confirmed that the high temperature incubation process had little influence on the morphology of dehydrated nanocomplexes.To our knowledge, the optimized operation condition is to hydrothermally incubate the Fc-F/GO hydrogels at 180 °C for 12 h. Subsequent thermal treatment at 600 °C leads to the incorporation of N species into the graphene lattice. The final product is a black monolithic hybrid aerogel composed of N-doped graphene networks and Fe3O4 NPs, which has a low density of 9.30 mg cm−3 (Fig. 1d and Table S1†). SEM images reveal that the obtained graphene sheets are thicker and more rigid (Fig. 3d and S2†). The mineralized Fe3O4 NPs significantly grew up (with sizes of 50–100 nm) and uniformly dispersed on the graphene surfaces. Such kind of local engagement of metal oxide NPs within graphene skeletons reinforces the interfacial contact between NPs and graphene layers. Therefore, the self-agglomerations of both NPs and graphene sheets are inhibited, which promoting the electrochemical activity and stability of the composites.48,49 The TEM characterization further validates the uniform distribution of Fe3O4 NPs on the graphene (Fig. 3e). Interestingly, this method can uniquely yield Fe3O4 single crystals on the graphene surface. High-resolution TEM (HRTEM) reveals a typical Fe3O4 NP with a single-crystalline texture, which is entirely encapsulated by graphene sheets, belongs to a cubic system with lattice parameters of a = 8.391 Å (Fig. 3f). The lattice observation demonstrates that the two orientations (indexed to the (111) surface and the (111) surface, respectively) with the same lattice spacing of 4.844 Å are rotated by 70.5° from one another. And the corresponding selected area electron diffraction (SAED) pattern recorded along (011) direction is shown in the inset (Fig. 3f).50 Meanwhile, the typical hexagonal graphene construction with a lattice spacing of 2.02 Å (indexed to the (002) facet) is also observed.
Fig. 4a–f show the high-angle annular dark field scanning TEM (HAADF-STEM) image and HAADF-STEM/energy-dispersive X-ray spectroscopy (HAADF-STEM-EDS) maps of the Fe3O4/N-GAs, respectively. It can be seen that the C and N elements are widely distributed all over the graphene surface, while the Fe and O elements are mainly located in the core of particles. Elemental distribution of a representative Fe3O4 NP was investigated by HAADF-STEM-EDS mapping, as shown in Fig. 5a–e. It is clear that Fe and O (light blue and dark blue) concentrate in the region where NPs are located, whereas C and N (red and green) spread everywhere. The Fe3O4/N-GAs nanocomposites were further analyzed by compositional linear scan with a higher spatial resolution across a single NP. Fig. 5f shows that C and N are evenly distributed across the whole surface, while Fe and O concenter on the core. Evidently, the N-doping sites and Fe3O4 are locally distributed on the graphene surface, which is crucial for the high catalytic performance towards ORR.26
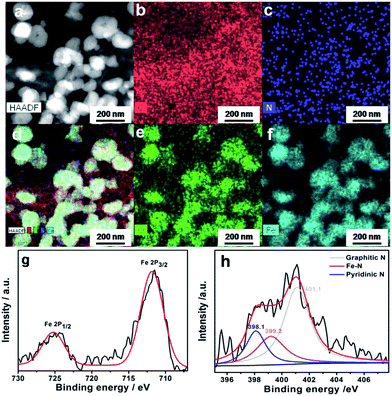 | ||
| Fig. 4 (a–f) The HAADF-STEM image, HAADF-STEM-EDS maps and (g) the XRD pattern of Fe3O4/N-GAs; XPS analysis of Fe3O4/N-GAs: (g) Fe 2p; (h) N 1s. | ||
 | ||
| Fig. 5 (a–e) The HAADF-STEM image, HAADF-STEM-EDS maps and (f) the cross-sectional compositional line-scanning profile of the single Fe3O4 nanocrystal. | ||
X-ray powder diffraction (XRD) and X-ray photoelectron spectrum (or spectra) (XPS) were also carried out to further identify the components of the oxidized products of supramolecular hydrogels. The XRD pattern confirms the existence of magnetic Fe3O4 NPs (JCPDS no. 65-3107) after thermal treatment at 600 °C (Fig. 6a). The diffraction peak at 20–30° with a d-spacing of 2.03 Å is assigned to the (002) surface of hexagonal graphene (2θ = 26°), which is similar to the HRTEM result of Fe3O4/N-GAs (d002 = 2.02 Å, Fig. 3f).51 These results agree well with previous reports that Fc moiety can be stoichiometrically oxidized to Fe3O4 by GO.46,47 Meanwhile, XPS exhibits clear Fe 2p signals corresponding to the binding energy of Fe 2p1/2 and Fe 2p3/2 for Fe3O4 at 725 and 711 eV, respectively (Fig. S3† and 4g). This is consistent with the XRD results, further confirming that Fe3O4 NPs have been effectively loaded on the surface of graphene. Besides, the high-resolution N 1s scan result reveals that the peaks of the N-doped graphene can be fitted to three main peaks of graphitic N (401.1 eV), Fe–N (399.2 eV) and pyridinic N (398.2 eV), coinciding with literature reports that graphitic N and pyridinic N could be formed through replacement of a C atom by a N atom in the graphene plane. At the same time, N could donate electrons to Fe at the high doping temperature. More importantly, those three N states are chemically active sites for ORR (Fig. 4h).13,17,52–55 The XPS of GO confirm the existence of two main types of carbon bonds: C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.6 eV) and C–O (286.6 eV), respectively (Fig. S4†).13,31 After reduction reaction, the most of oxygen-containing groups were removed and the peak associated with C
C (284.6 eV) and C–O (286.6 eV), respectively (Fig. S4†).13,31 After reduction reaction, the most of oxygen-containing groups were removed and the peak associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.6 eV) became predominant, while the peaks related to the oxidized carbon species were greatly weakened, confirming the reduction of GO into graphene (Fig. S5†). In addition, TEM EDS measurements also reveal that N atom has been doped in the aerogels (1.2 wt%, Fig. S6†).
C (284.6 eV) became predominant, while the peaks related to the oxidized carbon species were greatly weakened, confirming the reduction of GO into graphene (Fig. S5†). In addition, TEM EDS measurements also reveal that N atom has been doped in the aerogels (1.2 wt%, Fig. S6†).
 | ||
| Fig. 6 (a) The XRD pattern of Fe3O4/N-GAs; (b) CVs of Fe3O4/N-GAs and Pt/C for ORR in O2-saturated 0.1 M NaOH. | ||
Catalytic performance of Fe3O4/N-GAs
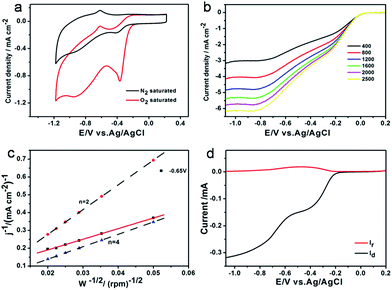
The electrocatalytic properties of Fe3O4/N-GAs for ORR were first studied by cyclic voltammetry (CV). Fig. 7a presents CVs of Fe3O4/N-GAs modified electrode in the 0.1 M NaOH solution with N2-saturated and O2-saturated, respectively. In the presence of O2, the onset potential is −0.18 V at the Fe3O4/N-GAs modified electrode, which is similar to that of commercial Pt/C (Fig. 6b).26,56 It is evidently that this material exhibits a significantly larger reduction current peak than Pt/C, implying an outstanding ORR catalytic performance. To discern the contribution of individual components and the possible synergistic effects among them, control experiments on N-GAs/GC, Fe3O4 NPs/GC electrodes were also carried out. As shown in Fig. S7,† the electrochemical reduction of O2 on the Fe3O4 NPs/GC electrode occurs at −0.21 V. In previous reports, Fe3O4 NPs possess intrinsic peroxidase property, which would play as mimic enzymes catalysis to improve the response towards O2 reduction.57–59 In addition, the increased surface area also plays an important role in the observed increased current. At the same time, the N-GAs modified electrode exhibits much better electrocatalytic activity. The electrochemical reduction of O2 already starts at −0.2 V at N-GAs/GC electrode. At approximately −0.40 V, a maximum reduction current of −0.60 mA cm−1 is observed, and at more negative potentials, the cathodic current shows a plateau. When Fe3O4 NPs was incorporated in the N-GAs, a cathodic current density which is much larger than that of the N-GAs/GC electrode is observed, and the onset potential is slight positive shift (about 20 mV). These results demonstrated that the Fe3O4/N-GAs/GC electrode exhibited the best response towards electrochemical reduction of O2. We propose that the increased cathodic current on the Fe3O4/N-GAs composite film electrode should be due to the synergistic effects between the N-GAs nanosheets and Fe3O4 NPs. Such observed synergy could be ascribed to the full contact between Fe3O4 and N-graphenes matrix, which improved electronic and ionic transport capacity and electron self-exchange in the film.11To quantify the stoichiometry of the catalytic oxygen reduction, voltammetric measurements at a rotating disk electrode (RDE) and a rotating ring-disk electrode (RRDE) were performed. Fig. 7b exhibits the current–potential curves of oxygen reduction in O2-saturated 0.1 M KOH solution at various rotation rates, respectively. It can be seen that one-step reduction pathway and potential-independent plateau currents are present at all rotation rates, also suggesting the synergistic effects between Fe3O4 NPs and N-GAs. Fig. 7c provides the Koutecky–Levich plot obtained from the plateau currents at −0.65 V, indicating that the ORR is a direct four-electron transfer process. A rotating GC disk-platinum ring electrode was further employed to determine the quantity of H2O2 that was generated by a two-electron reduction process of O2 at the disk electrode. Fig. 7d suggests that the ratio of the ring currents to disk currents (iR/iD) is 0.0081 for the Fe3O4/N-GAs complexes.60 According to iR/iD, the reaction electron number involved in the ORR is 3.95 (n = 4 − 2iR/(iDN)), where N is the current collection efficiency (0.37) of disk-platinum ring, being identical to that acquired from the Koutecky–Levich plot.61 The H2O yield can be estimated as following eqn (1):
 | (1) |
From our data, the calculated H2O yield on the Fe3O4/N-GAs catalysis during oxygen reduction is 95.7%, further confirming that this ORR follows the four-electron transfer pathway with a low H2O2 yield.
Conclusions
In summary, a facile but effective protocol was developed to fabricate well-organized hybrid aerogels of Fe3O4/N-doped graphene, which obtained directly from Fc-F/GO supramolecular hydrogels through in situ hydrothermal treatments. Importantly, since Fc-F hydrogelator is the single precursor for both Fe and N, the association force between two new born catalytical sites (Fe3O4 NPs and N-doped sites) should be much stronger than two different precursors, which results in an ideal electron transfer tunnel for the electrochemical catalytic process. Meanwhile, GO nanosheets with abundant functional groups are able to stabilize supramolecular hydrogels and subsequently facilitate the well-distribution of Fe3O4 NPs on ultrathin graphene films. Moreover, the in situ formed Fe3O4 NPs can inhibit the self-aggregation among graphene sheets during the reduction of GO, which would retain many channels to allow counterions in aqueous buffers to fastly move into or out of the films and greatly enhance the charge mobility within the films. Consequently, the as-prepared hybrid aerogels exhibit high efficiency in catalyzing the electrochemical ORR (comparing to commercial Pt/C). We expect that this report can offer a new route to produce the morphology-controlled inorganic NPs in hybrid graphene nanomaterials.Experimental details
Instrumentation
SEM images were obtained by a scanning electron microscope (FEI HELIOS NanoLab 600i, America). TEM images, HAADF-STEM images and HAADF-STEM-EDS maps were obtained from the TEM/STEM (FEI Titan G2 60-300, America), which includes corrector technologies enabling resolution of 80 pm. CD spectra measurements were used by a Jasco-815 CD spectrometer (Japan). A rotated rheometer used the AR 2000 ex TA Instrument (America). XRD measurements were performed on a D/max 2550 X-ray power diffractometer. XPS measurements were carried out at an ESCALAB 250Xi X-ray Photoelectron Spectrometer (ThermoFisher-VG Scientific, America). Voltammetry measurements were performed on the CHI 650D and CHI 832C electrochemical workstations (Shanghai, China). RDE tests were used by a Pine Modulated Speed Rotator (MSR, America) with rotation rates from 50–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm.
000 rpm.
Synthesis of Fe3O4/N-GAs
(a) Synthesis of Fc-F: synthesize Fc-F as the reference did.42(b) Synthesis of GO: synthesize GO as the reference did.62,63 Modified Hummers methods could yield well-distributed GO solution. However, to obtain ultrathin graphene is tricky and laborious, and we usually perform a 48 hours dialysis toward GO after reducing it. But, nonetheless, we still have the loss of 30% in the process of making N-GAs.
(c) The preparation of Fc-F/GO hybrid hydrogels: the lyophilized Fc-F was dissolved in DMSO (100 mg mL−1, as a stock solution). After the pH value of graphene oxide solution was adjusted to 7–8 by adding a small amount of PBS (10 mM, pH 9.0), the stock solution was diluted by the GO solution (a final concentration of 2 mg mL−1 or higher). The suspension turned to be a clear brown hydrogel after incubation for several minutes.
(d) Synthesis of Fe3O4/N-GAs: the Fe3O4/N-doped GO hydrogels were prepared by incubating Fc-F/GO hybrid hydrogels through hydrothermal treatment at 180 °C for 12 h. The as-prepared Fe3O4/N-doped GO hydrogels were directly dehydrated via a freeze-drying process to form 3D aerogels and then heated at 600 °C for 3 h under nitrogen protection to obtain high performance Fe3O4/N-GAs.
(e) Synthesis of Fe3O4 and N-GAs: synthesize Fe3O4 and N-GAs as the reference did.64,65
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21473257, 91127024). This work was also supported by the Tianshan scholarship from Xinjiang University and the Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, Jiangnan University, China (No. JDSJ2013-02).Notes and references
- Z. Niu, J. Chen, H. H. Hng, J. Ma and X. Chen, Adv. Mater., 2012, 24, 4144 CrossRef CAS PubMed.
- S. Yin, Y. Zhang, J. Kong, C. Zou, C. M. Li, X. Lu, J. Ma, F. Y. C. Boey and X. Chen, ACS Nano, 2011, 5, 3831 CrossRef CAS PubMed.
- S. Mao, G. Lu and J. Chen, Nanoscale, 2015, 7, 6924 RSC.
- Y. Y. Jiang, Y. Z. Lu, X. D. Wang, Y. Bao, W. Chen and L. Niu, Nanoscale, 2014, 6, 15066 RSC.
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed.
- M. Lefevre, E. Proietti, F. Jaouen and J. P. Dodelet, Science, 2009, 324, 71 CrossRef CAS PubMed.
- S. Guo, D. Li, H. Zhu, S. Zhang, N. M. Markovic, V. R. Stamenkovic and S. Sun, Angew. Chem., Int. Ed., 2013, 52, 3465 CrossRef CAS PubMed.
- W. Liu, P. Rodriguez, L. Borchardt, A. Foelske, J. P. Yuan, A. K. Herrmann, D. Geiger, Z. K. Zheng, S. Kaskel, N. Gaponik, R. Kotz, T. J. Schmidt and A. Eychmuller, Angew. Chem., Int. Ed., 2013, 52, 9849 CrossRef CAS PubMed.
- S. Guo and S. Sun, J. Am. Chem. Soc., 2012, 134, 2492 CrossRef CAS PubMed.
- D. Wen, A. K. Herrmann, L. Borchardt, F. Simon, W. Liu, S. Kaskel and A. Eychmuller, J. Am. Chem. Soc., 2014, 136, 2727 CrossRef CAS PubMed.
- G. Wu, N. H. Mack, W. Gao, S. Ma, R. Zhong, J. Han, J. K. Baldwin and P. Zelenay, ACS Nano, 2012, 6, 9764 CrossRef CAS PubMed.
- C. K. Chua, A. Ambrosi, Z. Sofer, A. Macková, V. Havránek, I. Tomandl and M. Pumera, Chem.–Eur. J., 2014, 20, 15760 CrossRef CAS PubMed.
- S. A. Wohlgemuth, R. J. White, M. G. Willinger, M. M. Titirici and M. Antonietti, Green Chem., 2012, 14, 1515 RSC.
- Z. Juanjuan, L. Ruiyi, L. Zaijun, L. Junkang, G. Zhiguo and W. Guangli, Nanoscale, 2014, 6, 5458 RSC.
- C. Wang, Y. Li, X. He, Y. Ding, Q. Peng, W. Zhao, E. Shi, S. Wu and A. Cao, Nanoscale, 2015, 7, 7550 RSC.
- W. Wei, S. B. Yang, H. X. Zhou, I. Lieberwirth, X. L. Feng and K. Mullen, Adv. Mater., 2013, 25, 2909 CrossRef CAS PubMed.
- S. Yang, X. Feng, X. Wang and K. Müllen, Angew. Chem., Int. Ed., 2011, 50, 5339 CrossRef CAS PubMed.
- J. Luo, J. Liu, Z. Zeng, C. F. Ng, L. Ma, H. Zhang, J. Lin, Z. Shen and H. J. Fan, Nano Lett., 2013, 13, 6136 CrossRef CAS PubMed.
- Y. Zhao, C. G. Hu, Y. Hu, H. H. Cheng, G. Q. Shi and L. T. Qu, Angew. Chem., Int. Ed., 2012, 51, 11371 CrossRef CAS PubMed.
- Y. Zhu, B. Zhang, X. Liu, D. Wang and D. S. Su, Angew. Chem., Int. Ed., 2014, 53, 10673 CrossRef CAS PubMed.
- G. Zhou, D. Wang, F. Li, L. Zhang, N. Li, Z. Wu, L. Wen, G. Lu and H. Cheng, Chem. Mater., 2010, 22, 5306 CrossRef CAS.
- J. Xu, P. Gao and T. S. Zhao, Energy Environ. Sci., 2012, 5, 5333 CAS.
- Y. Meng, W. Song, H. Huang, Z. Ren, S. Chen and S. L. Suib, J. Am. Chem. Soc., 2014, 136, 11452 CrossRef CAS PubMed.
- H. T. Chung, J. H. Won and P. Zelenay, Nat. Commun., 2013, 4, 1922 CrossRef PubMed.
- X. W. Lou, D. Deng, J. Y. Lee, J. Feng and L. A. Archer, Adv. Mater., 2008, 20, 258 CrossRef CAS PubMed.
- Z. Wu, S. Yang, Y. Sun, K. Parvez, X. Feng and K. Müllen, J. Am. Chem. Soc., 2012, 134, 9082 CrossRef CAS PubMed.
- M. Sun, H. Liu, Y. Liu, J. Qu and J. Li, Nanoscale, 2015, 7, 1250 RSC.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Nat. Mater., 2011, 10, 780 CrossRef CAS PubMed.
- L. Li, H. B. Wu, L. Yu, S. Madhavi and X. W. Lou, Adv. Mater. Interfaces, 2014 DOI:10.1002/admi.201400050.
- H. Li, L. Liu and F. Yang, J. Mater. Chem., 2013, 1, 3446 RSC.
- W. Chen, S. Li, C. Chen and L. Yan, Adv. Mater., 2011, 23, 5679 CrossRef CAS PubMed.
- Z. Wu, Y. Sun, Y. Tan, S. Yang, X. Feng and K. Müllen, J. Am. Chem. Soc., 2012, 134, 19532 CrossRef CAS PubMed.
- S. Chen and S. Qiao, ACS Nano, 2013, 7, 10190 CrossRef CAS PubMed.
- X. Wang, W. Tian, D. Liu, C. Zhi, Y. Bando and D. Golberg, Nano Energy, 2013, 2, 257 CrossRef CAS PubMed.
- Y. Liang, H. Wang, J. Zhou, Y. Li, J. Wang, T. Regier and H. Dai, J. Am. Chem. Soc., 2012, 134, 3517 CrossRef CAS PubMed.
- X. Y. Zhang, X. Chen, K. J. Zhang, S. P. Pang, X. H. Zhou, H. X. Xu, S. M. Dong, P. X. Han, Z. Y. Zhang, C. J. Zhang and G. L. Cui, J. Mater. Chem. A, 2013, 1, 3340 CAS.
- J. Duan, S. Chen, S. Dai and S. Z. Qiao, Adv. Funct. Mater., 2014, 24, 2072 CrossRef CAS PubMed.
- S. Bag, K. Roy, C. S. Gopinath and C. R. Raj, ACS Appl. Mater. Interfaces, 2014, 6, 2692 CAS.
- L. Barišić, M. Čakić, K. A. Mahmoud, Y. Liu, H. B. Kraatz, H. Pritzkow, S. I. Kirin, N. Metzler Nolte and V. Rapić, Chem.–Eur. J., 2006, 12, 4965 CrossRef PubMed.
- S. I. Kirin, H. B. Kraatz and N. Metzler Nolte, Chem. Soc. Rev., 2006, 35, 348 RSC.
- X. Yan, P. Zhu and J. Li, Chem. Soc. Rev., 2010, 39, 1877 RSC.
- Z. F. Sun, Z. Y. Li, Y. H. He, R. J. Shen, L. Deng, M. H. Yang, Y. Z. Liang and Y. Zhang, J. Am. Chem. Soc., 2013, 135, 13379 CrossRef CAS PubMed.
- S. Debnath, S. Roy and R. V. Ulijn, J. Am. Chem. Soc., 2013, 135, 16789 CrossRef CAS PubMed.
- S. Fleming, S. Debnath, P. W. J. M. Frederix, N. T. Hunt and R. V. Ulijn, Biomacromolecules, 2014, 15, 1171 CrossRef CAS PubMed.
- W. Miao, D. Yang and M. Liu, Chem.–Eur. J., 2015, 21, 7562 CrossRef CAS PubMed.
- H. Cong, X. Ren, P. Wang and S. Yu, ACS Nano, 2012, 6, 2693 CrossRef CAS PubMed.
- Z. Fan, W. Kai, J. Yan, T. Wei, L. Zhi, J. Feng, Y. Ren, L. Song and F. Wei, ACS Nano, 2010, 5, 191 CrossRef PubMed.
- X. H. Cao, B. Zheng, X. H. Rui, W. H. Shi, Q. Y. Yan and H. Zhang, Angew. Chem., Int. Ed., 2014, 53, 1404 CrossRef CAS PubMed.
- H. Cong, J. Chen and S. Yu, Chem. Soc. Rev., 2014, 43, 7295 RSC.
- Y. Y. Ma, Q. Kuang, Z. Y. Jiang, Z. X. Xie, R. B. Huang and L. S. Zheng, Angew. Chem., Int. Ed., 2008, 47, 8901 CrossRef CAS PubMed.
- W. Ding, Z. Wei, S. Chen, X. Qi, T. Yang, J. Hu, D. Wang, L. Wan, S. F. Alvi and L. Li, Angew. Chem., 2013, 125, 11971 CrossRef PubMed.
- Z. Wu, A. Winter, L. Chen, Y. Sun, A. Turchanin, X. Feng and K. Müllen, Adv. Mater., 2012, 24, 5130 CrossRef CAS PubMed.
- L. Lin, Q. Zhu and A. Xu, J. Am. Chem. Soc., 2014, 136, 11027 CrossRef CAS PubMed.
- G. Wu, C. M. Johnston, N. H. Mack, K. Artyushkova, M. Ferrandon, M. Nelson, J. S. Lezama Pacheco, S. D. Conradson, K. L. More, D. J. Myers and P. Zelenay, J. Mater. Chem., 2011, 21, 11392 RSC.
- H. Yin, C. Zhang, F. Liu and Y. Hou, Adv. Funct. Mater., 2014, 24, 2930 CrossRef CAS PubMed.
- A. Shen, Y. Zou, Q. Wang, R. A. W. Dryfe, X. Huang, S. Dou, L. Dai and S. Wang, Angew. Chem., Int. Ed., 2014, 53, 10804 CrossRef CAS PubMed.
- J. Gao, H. Gu and B. Xu, Acc. Chem. Res., 2009, 42, 1097 CrossRef CAS PubMed.
- H. Wei and E. Wang, Anal. Chem., 2008, 80, 2250 CrossRef CAS PubMed.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577 CrossRef CAS PubMed.
- M. Huang, Y. Shao, X. Sun, H. Chen, B. Liu and S. Dong, Langmuir, 2004, 21, 323 CrossRef PubMed.
- S. Liu, J. Xu, H. Sun and D. Li, Inorg. Chim. Acta, 2000, 306, 87 CrossRef CAS.
- Y. Liu, Y. Zhang, G. Ma, Z. Wang, K. Liu and H. Liu, Electrochim. Acta, 2013, 88, 519 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- H. Hu, Z. Zhao, W. Wan, Y. Gogotsi and J. Qiu, Adv. Mater., 2013, 25, 2219 CrossRef CAS PubMed.
- L. Y. Wang, J. Bao, L. Wang, F. Zhang and Y. D. Li, Chem.–Eur. J., 2006, 12, 6341 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional electron microscopy images, CV curves and so on. See DOI: 10.1039/c5ra15595a |
| This journal is © The Royal Society of Chemistry 2015 |