Synthesis of functionalized poly(vinyl acetate) mediated by alkyne-terminated RAFT agents†
Joana. R.
Góis
a,
Anatoliy V.
Popov
b,
Tamaz
Guliashvili
a,
Arménio C.
Serra
 a and
Jorge F. J.
Coelho
a and
Jorge F. J.
Coelho
 *a
*a
aDepartment of Chemical Engineering, University of Coimbra, Polo II, Rua Sílvio Lima, 3030-790 Coimbra, Portugal. E-mail: jcoelho@eq.uc.pt; Fax: +351 239 798 703; Tel: +351 239 798 744
bDepartment of Radiology, University of Pennsylvania, Philadelphia, PA19104, USA
First published on 15th October 2015
Abstract
Two new xanthates with alkyne functionalities were synthesized for the reversible addition fragmentation chain transfer (RAFT) polymerization of vinyl acetate (VAc). The new RAFT agents were fully characterized by 1H and 13C NMR spectroscopy. Unlike the alkyne terminated RAFT agent (AT-X1) the protected alkyne-terminated RAFT agent (PAT-X1) was able to conduct the RAFT polymerization of VAc with a good control over the molecular weight (MW) and relatively narrow MW distributions (Đ < 1.4). The linear evolution of Mn with conversion as well as the close agreement between Mn,th and Mn,GPC values confirmed the controlled features of the RAFT system. It is worth mentioning that the polymer dispersity remained very low (Đ < 1.20) until relatively high monomer conversions (60%) due to the non-activated nature of VAc. The chain end-functionality of the obtained polymers was evaluated by 1H NMR, FTIR-ATR and UV-Vis absorption analysis. The “livingness” of the obtained polymer was confirmed by a successful chain extension experiment. The deprotection of the alkyne functionality in the PVAc, allowed a further copper catalyzed azide–alkyne [3 + 2] dipolar cycloaddition reaction (CuAAC) with an azido terminated-poly(ethylene glycol) (PEG-N3), to afford PVAc–PEG block-copolymers as a proof-of-concept.
The reversible deactivation radical polymerization (RDRP) has witnessed enormous improvements during the last two decades.1–5 For RDRP of activated monomers such as acrylates, methacrylates or styrene, different modifications of atom transfer radical polymerization (ATRP) have successfully been applied.6–10 However, the development of new RDRP systems that are able to polymerize such non-activated monomers, such as vinyl chloride,11–13N-vinyl pyrrolidone14 or vinyl acetate (VAc)15,16 remains an important challenge. The reversible addition–fragmentation chain transfer (RAFT) polymerization is considered a very effective RDRP method for the polymerization of less activated monomers.12,17 Other RDRP methods mediated by transition metal catalysts,18,19 iodine transfer11,20 or nitroxides21 have been studied for vinyl chloride and VAc. Recently, some authors reported the use of cobalt complex as organic metallic complexes for the controlled synthesis of poly(vinyl acetate) (PVAc).22–25 VAc stands out as one of the most studied non-activated vinyl monomers.26 PVAc has a wide range of industrial applications, from paints to coatings as well as its hydrolyzed derivative, poly(vinyl alcohol) (PVA), that extends its applications to the biomedical field.27
The choice of the RAFT agent, is of outmost importance for the success of this RDRP method.28,29 For non-activated monomers, xanthate mediated RAFT polymerization30 is one of the most straightforward RDRP strategies to afford optimal control over the polymerization. Due to the high reactivity of the VAc propagating radical, the RAFT agent should have an efficient leaving group, with similar reactivity to the growing macroradical towards the monomer addition. Most of the literature reports, concerning the xanthate mediated RAFT polymerization of VAc, refer the use of methyl(ethoxy carbonyl sulfanyl acetate) (Fig. 1 (Y1)),31–33 methyl 2-((ethoxycarbonothiol)thio)propanoate (also known as O-ethyl-S-(1-methoxy carbonyl) ethyl dithiocarbonate), with 2-propionyl moiety as the leaving group (Fig. 1 (X1)), for either bulk,34 solution35,36 or miniemulsion polymerizations,33 or very similar RAFT agents.37,38 Nevertheless, other RAFT agents such as dithiocarbamates have also been reported for the well-defined polymerization of VAc.39,40
The conjugation of polymers through a post polymerization coupling strategy is also a powerful tool in macromolecular engineering to afford new block copolymers with segments that are impossible to link by direct copolymerization. In fact, the single step synthesis of block copolymers is limited to few monomers functionalities, usually with similar chemical and physical properties, or require the use of specific RAFT agents based on a switchable dithiocarbamate moiety that are able to mediate the polymerization of both activated and non-activated monomers.41 In the case of non-activated monomers, where controlled copolymerization is more difficult, reactions inspired by the “click” coupling approach is a convenient strategy to achieve new polymer architectures, otherwise difficult to access.42–44 The direct polymerization using a “clickable” functionalized RAFT agent is a common strategy to afford polymers with a specific chain-end functionality, without the need of post-modification procedures.45 CuAAC reaction between azide and alkyne chain-end functionalities is one of the most explored “click” reaction.46 Stenzel and co-workers reported the facile synthesis of poly(styrene)-b-PVAc block copolymers through a “click” coupling approach from a PVAc synthesized using an azido-xanthate RAFT agent and poly(styrene) synthesized using one alkyne-dithiobenzoate agent.45 A similar strategy was proposed, by the same group, for the synthesis of comb-like copolymers, from the reaction of an azido functionalized linear PVAc with one alkyne modified methacrylate monomer.47 The literature involving the use of xanthates with alkyne functionality is very scarce.
To the best of our knowledge, only one reference is available describing the synthesis of (S)-2-(propynyl propionate)-O-ethyl xanthate, an alkyne terminated RAFT agent, for the controlled synthesis of N-vinylpyrrolidone.48 The aim of the present study was to synthesize new efficient alkyne functionalized xanthate RAFT agents, able to conduct controlled polymerization of vinyl acetate. The present strategy enabled the straightforward preparation of PVAc block copolymers through a simple post polymerization coupling method.
Experimental
Materials
Vinyl acetate (VAc) (≥99%, Aldrich) was purified by passing the monomer through a basic alumina column and then distilled under vacuum (bp 72–73 °C). 2,2′-Azobis(2-methylpropionitrile) (AIBN) (98%, Fluka) was purified by recrystallization from methanol before use. 1,4-Dioxane (99.8%, Acros Organics) was passed through alumina column to remove peroxides and distilled under reduced pressure prior to use. Poly(ethylene glycol) methyl ether (mPEG113, MW = 5000 Da) (Aldrich) was dried by azeotropic distillation with toluene. Sodium ascorbate (NaAsc) (≥98%, Sigma), copper(II) sulfate pentahydrate (CuSO4·5H2O) (≥98%, Aldrich), methyl 2-bromopropionate (98%, Aldrich), methanol (99.9%, Fisher Scientific), dichloromethane (DCM) (99.99%, Fisher Scientific), potassium ethyl xanthogenate (96%; Aldrich) anhydrous sodium sulfate (≥98%, Fisher Chemical), ethyl acetate (≥99.5%, Fisher Scientific), diethyl ether (99.85%, Fisher Scientific), hexane (99.05%, Fisher Scientific), thionyl chloride (≥99%, Sigma-Aldrich), propargyl alcohol (PA) (99%, Aldrich), triethylamine (≥99%, Sigma-Aldrich), 2-bromopropionic acid (≥99%, Aldrich), 5-trimethylsilyl-4-pentyn-1-ol (96%; Aldrich), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (≥99.0%, Sigma), 4-(dimethylamino)pyridine (≥99%, Aldrich), tetrabutyl ammonium fluoride trihydrate (TBAF·3H2O) (99%, Acros Organics), sodium azide (99%; Aldrich), tetrahydrofuran (THF) (≥99.9%, Sigma-Aldrich), dimethylformamide (DMF) (≥99.8%; Sigma-Aldrich), deuterated chloroform (CDCl3) (≥99.8%, Euriso-top, +1% TMS) and deuterated DCM (CD2Cl2) (≥99.6%, Euriso-top) were used as received. Azido terminated-poly(ethylene glycol) (mPEG113-N3) was synthesized by a nucleophilic substitution of poly(ethylene glycol) methyl ether bromoisobutyrate (mPEG113-BiB) (previously synthesized according to the literature procedures49) using NaN3 in DMF (procedure adapted from literature50) (see ESI† for detailed synthesis).Techniques
The 1H and 13C NMR spectra were recorded on a Bruker DMX-360 (360 MHz for 1H NMR and 90 MHz for 13C NMR, with a 5 mm manual switching QNP) and a Bruker Avance III spectrometer (400 MHz, with a 5 mm TIX triple resonance detection probe), in a deuterated solvent. Monomer conversions were determined by integration of monomer and polymer peaks using MestRenova software version: 6.0.2–5475. Fourier-transform infrared spectroscopy (FTIR) was performed at 64 scans and with a 4 cm−1 resolution between 500 and 3500 cm−1, using a JASCO 4200 FTIR spectrometer, operating in the ATR mode (MKII GoldenGate™ Single Reflexion ATR System). The thermogravimetric analysis (TGA) was carried out on a TGA Q 500 machine (TA Instruments) with a heating ramp set at a constant 10 °C min−1, and covering a temperature range from 25 to 300 °C. High performance gel permeation chromatography (HPSEC) was performed using a Viscotek (ViscotekTDAmax) with a differential viscometer (DV), right-angle laser-light scattering (RALLS, Viscotek), and refractive index (RI) detectors, using column set of a PL 10 μm guard column followed by one MIXED-E PLgel column and one MIXED-C PLgel column. Filtered THF was used as an eluent at a flow rate of 1 mL min−1 at 30 °C. The samples were filtered through a polytetrafluoroethylene membrane with 0.2 μm pore before injection and the system was calibrated with narrow PS standards. The dn/dc of PVAc in THF at 30 °C was determined as 0.0581 (for λ = 670 nm) using a RUDOLPH RESEARCH J357 Automatic Refractometer (J357-NDS-670-CC). Molecular weight (Mn,GPC) and dispersity (Đ) of synthesized polymers were determined by using either a universal calibration or multidetector analysis (OmniSEC software version: 4.6.1.354). Ultraviolet-Visible (UV-Vis) spectroscopy was carried out using a Jasco V-530 spectrophotometer. The analyses were carried out in CHCl3 in the 250–400 nm range at 25 °C. Absorption spectra were measured from 250 to 400 nm with a resolution of 2.0 nm in a 10 mm UV-cuvette.Procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol%), washed three times with water and dried over anhydrous sodium sulfate. The solvent was evaporated at reduced pressure to give a yellow liquid that was further purified by column chromatography on silica with hexane/ethyl acetate (10
1 vol%), washed three times with water and dried over anhydrous sodium sulfate. The solvent was evaporated at reduced pressure to give a yellow liquid that was further purified by column chromatography on silica with hexane/ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol%) as the eluent to give X1 (4.30 g, 67%). 1H NMR (360 MHz, CDCl3): δ (ppm) 4.6232 (q, 1H, 3JHH = 7.14 Hz, diastereotopic –OCHHCH3), 4.6199 (q, 1H, 3JHH = 7.1 Hz, diastereotopic –OCHHCH3), 4.36 (q, 1H, 3JHH = 7.4 Hz, –CH), 3.73 (s, 3H, –CH3), 1.56 (d, 3H, 3JHH = 7.4 Hz, –CHCH3), 1.40 (t, 3H, JHH = 7.14 Hz, –CH2CH3).
1 vol%) as the eluent to give X1 (4.30 g, 67%). 1H NMR (360 MHz, CDCl3): δ (ppm) 4.6232 (q, 1H, 3JHH = 7.14 Hz, diastereotopic –OCHHCH3), 4.6199 (q, 1H, 3JHH = 7.1 Hz, diastereotopic –OCHHCH3), 4.36 (q, 1H, 3JHH = 7.4 Hz, –CH), 3.73 (s, 3H, –CH3), 1.56 (d, 3H, 3JHH = 7.4 Hz, –CHCH3), 1.40 (t, 3H, JHH = 7.14 Hz, –CH2CH3).
2-Bromopropionyl chloride. 4.00 mL of thionyl chloride (5.14 mmol) was slowly added to 4.50 mL of 2-bromopropionic acid (50.00 mmol). A small amount of DMF (10 μL) was used to catalyze the reaction. The mixture was heated to 80 °C and stirred until the complete release of gaseous by-products of the reaction. The product was used without any further purification steps.
(RS)-Propargyl 2-bromopropionate. A solution of PA (2.80 g, 50.00 mmol) and triethylamine (5.06 g, 50.00 mmol) in DCM was cooled down to ∼−20 °C with liquid nitrogen. The 2-bromopropionyl chloride was added in portions to the solution. The solution was left off from the cold and kept under stirring until reach the room temperature. The product was washed three times with water and the organic phase was dried under anhydrous sodium sulfate. After solvent evaporation the product was obtained as a light yellow oil (6.98 g, 73%). 1H NMR (360 MHz, CDCl3): δ (ppm) 4.7599 (d, 1H, 4JHH = 2.5 Hz, diastereotopic HC
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CHHO–), 4.7524 (d, 1H, 4JHH = 2.5 Hz, diastereotopic HC
C–CHHO–), 4.7524 (d, 1H, 4JHH = 2.5 Hz, diastereotopic HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CHHO–), 4.39 (q, 1H, 3JHH = 6.9 Hz, –CH–Br), 2.5 (t, 1H, JHH = 2.5 Hz, HC
C–CHHO–), 4.39 (q, 1H, 3JHH = 6.9 Hz, –CH–Br), 2.5 (t, 1H, JHH = 2.5 Hz, HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–), 1.82 (d, 3H, 3JHH = 6.9 Hz, –CH3). 13C NMR (90 MHz, CDCl3): δ (ppm) 169.48 (C
C–), 1.82 (d, 3H, 3JHH = 6.9 Hz, –CH3). 13C NMR (90 MHz, CDCl3): δ (ppm) 169.48 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 76.86 (
O), 76.86 (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–), 75.74 (HC
C–), 75.74 (HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) ), 53.40 (CH2–O), 39.30 (CH–Br), 21.58 (Br–CH3).
), 53.40 (CH2–O), 39.30 (CH–Br), 21.58 (Br–CH3).
AT-X1. Propargyl 2-bromopropionate (4.04 g, 21.13 mmol) was dissolved in 25 mL of PA and the solution was cooled down in an ice bath. Potassium ethyl xanthogenate (3.84 g, 23.92 mmol) was then added to the solution in portions over a period of 30 minutes. After the complete dissolution of the salt, the reaction mixture was stirred at room temperature overnight. The solid KBr was filtered off, the filtrate was extracted with ether/hexane (2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol%) and the extract was washed three times with water (500 mL) and dried over anhydrous sodium sulfate. The solvent was evaporated at reduced pressure to give a yellow liquid that was further purified by column chromatography on silica starting with hexane and then hexane/ethyl acetate (10
1 vol%) and the extract was washed three times with water (500 mL) and dried over anhydrous sodium sulfate. The solvent was evaporated at reduced pressure to give a yellow liquid that was further purified by column chromatography on silica starting with hexane and then hexane/ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol%) as the eluent to give AT-X1 as a yellow liquid (2.64 g, 54%). 1H NMR (360 MHz, CDCl3): δ (ppm) 4.7335 (d, 1H, 4JHH = 2.4 Hz, diastereotopic
1 vol%) as the eluent to give AT-X1 as a yellow liquid (2.64 g, 54%). 1H NMR (360 MHz, CDCl3): δ (ppm) 4.7335 (d, 1H, 4JHH = 2.4 Hz, diastereotopic ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CHH–O), 4.7321 (d, 1H, 4JHH = 2.4 Hz, diastereotopic
C–CHH–O), 4.7321 (d, 1H, 4JHH = 2.4 Hz, diastereotopic ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CHH–O), 4.6310 (q, 1H, 3JHH = 7.1 Hz, diastereotopic CHH–CH3), 4.6302 (q, 1H, 3JHH = 7.1 Hz, diastereotopic CHH–CH3), 4.41 (q, 1H, 3JHH = 7.4 Hz, CH–CH3), 2.49 (t, 1H, 4JHH = 2.4 Hz, CH
C–CHH–O), 4.6310 (q, 1H, 3JHH = 7.1 Hz, diastereotopic CHH–CH3), 4.6302 (q, 1H, 3JHH = 7.1 Hz, diastereotopic CHH–CH3), 4.41 (q, 1H, 3JHH = 7.4 Hz, CH–CH3), 2.49 (t, 1H, 4JHH = 2.4 Hz, CH![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 1.58 (d, 3H, 3JHH = 7.4 Hz, CH3–CH), 1.41 (t, 3H, 3JHH = 7.1 Hz, CH3–CH2). 13C NMR (90 MHz, CDCl3): δ (ppm) 211.69 (C
C), 1.58 (d, 3H, 3JHH = 7.4 Hz, CH3–CH), 1.41 (t, 3H, 3JHH = 7.1 Hz, CH3–CH2). 13C NMR (90 MHz, CDCl3): δ (ppm) 211.69 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), 170.79 (C
S), 170.79 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 77.16 (
O), 77.16 (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–), 75.46 (HC
C–), 75.46 (HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) ), 70.44 (CH2–CH3), 53.13 (CH2–O), 46.83 (CH–CH3), 16.68 (CH3–CH), 13.73 (CH3–CH2) (the peak at 77.16 ppm is overlapped with the solvent peak). 13C NMR (90 MHz, CD2Cl2): (ppm) 212.14 (C
), 70.44 (CH2–CH3), 53.13 (CH2–O), 46.83 (CH–CH3), 16.68 (CH3–CH), 13.73 (CH3–CH2) (the peak at 77.16 ppm is overlapped with the solvent peak). 13C NMR (90 MHz, CD2Cl2): (ppm) 212.14 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), 171.12 (C
S), 171.12 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 77.74 (
O), 77.74 (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–), 75.64 (HC
C–), 75.64 (HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) ), 71.14 (CH2–CH3), 53.54 (CH2–O), 47.14 (CH–CH3), 16.96 (CH3–CH), 13.97 (CH3–CH2).
), 71.14 (CH2–CH3), 53.54 (CH2–O), 47.14 (CH–CH3), 16.96 (CH3–CH), 13.97 (CH3–CH2).
2-(Ethoxycarbonothioylthio)propanoic acid. 4.18 g (27.30 mmol) of 2-bromopropionic acid was dissolved in 40 mL of dry methanol. The solution was cooled down in an ice bath. Potassium ethyl xanthogenate 5.126 g (31.98 mmol) was slowly added to the methanol solution, in portions, over a period of 30 min. After the complete dissolution of potassium ethyl xanthogenate, the ice bath was removed and the reaction proceeded at room temperature for 24 h. The reaction by-product, KBr, was filtered under reduced pressure and the product extracted with an ether/hexane mixture (2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol%), washed three times with water and dried over anhydrous sodium sulfate. The product was obtained after the solvent evaporation at reduced pressure. 1H NMR (400 MHz, CDCl3): δ (ppm) 10.00 (s, 1H, –OH), 4.6419 (q, 1H, 3JHH = 7.1 Hz, diastereotopic CHH–CH3), 4.6399 (q, 1H, 3JHH = 7.1 Hz, diastereotopic CHH–CH3), 4.41 (q, 1H, 3JHH = 7.47 Hz, –CH–CH3), 1.60 (d, 3H, 3JHH = 7.4 Hz, –CH3–CH), 1.41 (t, 3H, 3JHH = 7.1 Hz, –CH3–CH2).
1 vol%), washed three times with water and dried over anhydrous sodium sulfate. The product was obtained after the solvent evaporation at reduced pressure. 1H NMR (400 MHz, CDCl3): δ (ppm) 10.00 (s, 1H, –OH), 4.6419 (q, 1H, 3JHH = 7.1 Hz, diastereotopic CHH–CH3), 4.6399 (q, 1H, 3JHH = 7.1 Hz, diastereotopic CHH–CH3), 4.41 (q, 1H, 3JHH = 7.47 Hz, –CH–CH3), 1.60 (d, 3H, 3JHH = 7.4 Hz, –CH3–CH), 1.41 (t, 3H, 3JHH = 7.1 Hz, –CH3–CH2).
PAT-X1. In a 200 mL flask, the 2-(ethoxycarbonothioylthio) propanoic acid (0.60 g, 3.10 mmol) was dissolved in 60 mL of dry DCM. 5-Trimethylsilyl-4-pentyn-1-ol (0.68 mL, 3.74 mmol) was added and the mixture was cooled down to 0 °C and bubbled with argon. N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydro-chloride (0.78 g, 4.09 mmol) and 4-(dimethylamino)pyridine (5.76 mg, 0.05 mmol) were then added to the solution and the mixture was stirred in the ice bath for more 30 min. The reaction was left at room temperature for 24 h. The RAFT agent was purified by column chromatography on silica with hexane/ethyl acetate (10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol%) as the eluent. The PAT-X1 was obtained as a yellow oil (0.89 g, 86.6%). 1H NMR (400 MHz, CDCl3): δ (ppm) 4.64 (q, 2H, 3JHH = 7.12 Hz; CH2–CH3), 4.38 (q, 1H, 3JHH = 7.30 Hz, –CH–CH3), 4.23 (t, 2H, 3JHH = 6.28 Hz; CH2–O), 2.33 (t, 2H, 3JHH = 7.05 Hz; C
1 vol%) as the eluent. The PAT-X1 was obtained as a yellow oil (0.89 g, 86.6%). 1H NMR (400 MHz, CDCl3): δ (ppm) 4.64 (q, 2H, 3JHH = 7.12 Hz; CH2–CH3), 4.38 (q, 1H, 3JHH = 7.30 Hz, –CH–CH3), 4.23 (t, 2H, 3JHH = 6.28 Hz; CH2–O), 2.33 (t, 2H, 3JHH = 7.05 Hz; C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH2–), 1.87 (m, 2H, 3JHH = 6.66 Hz; –CH2–), 1.57 (d, 3H, 3JHH = 7.14 Hz, –CH3–CH), 1.42 (t, 3H, 3JHH = 7.13 Hz, –CH3–CH2), 0.14 (s, 9H, (CH3)3–Si). 13C NMR (90 MHz, CDCl3) δ (ppm) 212.22 (C
C–CH2–), 1.87 (m, 2H, 3JHH = 6.66 Hz; –CH2–), 1.57 (d, 3H, 3JHH = 7.14 Hz, –CH3–CH), 1.42 (t, 3H, 3JHH = 7.13 Hz, –CH3–CH2), 0.14 (s, 9H, (CH3)3–Si). 13C NMR (90 MHz, CDCl3) δ (ppm) 212.22 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), 171.48 (C
S), 171.48 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 105.60 (
O), 105.60 (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–O), 85.63 (Si–C
C–O), 85.63 (Si–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) ), 70.41 (CH2–CH3), 64.45 (CH2–O), 47.33 (CH–CH3), 27.71 (CH2–CH2–CH2), 17.01 (
), 70.41 (CH2–CH3), 64.45 (CH2–O), 47.33 (CH–CH3), 27.71 (CH2–CH2–CH2), 17.01 (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH2–), 16.65 (CH3–CH), 13.83 (CH3–CH2), 0.22 ((CH3)3).
C–CH2–), 16.65 (CH3–CH), 13.83 (CH3–CH2), 0.22 ((CH3)3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.2 in 1,4-dioxane.
VAc (2.02 g, 23.49 mmol), X1 (48.47 mg, 0.23 mmol), AIBN (7.82 mg, 0.05 mg) and 1.4-dioxane (1.93 mL; previously bubbled with nitrogen for about 10 min) were placed into a 25 mL Schlenk reactor. The reactor was sealed, frozen in liquid nitrogen, and the mixture was deoxygenated with four freeze–vacuum–thaw cycles and purged with nitrogen. The Schlenk reactor was placed in an oil bath at 60 °C with stirring (500 rpm). Different reaction mixture samples were collected during the polymerization through an airtight syringe, purging the side arm of the Schlenk reactor with nitrogen. The collected samples were analyzed by 1H NMR spectroscopy to calculate the monomer conversion and theoretical molecular weight (Mn,th), and by GPC to determine Mn,GPC and Đ of the polymers. The other RAFT polymerizations were carried out employing the same procedure described but using AT-X1 or PAT-X1 as the RAFT agents.
0.2 in 1,4-dioxane.
VAc (2.02 g, 23.49 mmol), X1 (48.47 mg, 0.23 mmol), AIBN (7.82 mg, 0.05 mg) and 1.4-dioxane (1.93 mL; previously bubbled with nitrogen for about 10 min) were placed into a 25 mL Schlenk reactor. The reactor was sealed, frozen in liquid nitrogen, and the mixture was deoxygenated with four freeze–vacuum–thaw cycles and purged with nitrogen. The Schlenk reactor was placed in an oil bath at 60 °C with stirring (500 rpm). Different reaction mixture samples were collected during the polymerization through an airtight syringe, purging the side arm of the Schlenk reactor with nitrogen. The collected samples were analyzed by 1H NMR spectroscopy to calculate the monomer conversion and theoretical molecular weight (Mn,th), and by GPC to determine Mn,GPC and Đ of the polymers. The other RAFT polymerizations were carried out employing the same procedure described but using AT-X1 or PAT-X1 as the RAFT agents.
Results and discussion
Synthesis of the RAFT agents
The use of functionalized RAFT agents avoids further steps involving the modification of the terminals in the polymeric chain structures with chemical groups suitable for further reactions, namely reactions inspired by the “click” coupling strategies. Two different RAFT agents for the polymerization of non-activated monomers were synthesized (AT-X1 and PAT-X1), based on O-ethyl-S-(1-methoxycarbonyl) ethyl-dithiocarbonate xanthate (X1).34–36 The structures of the RAFT agents are present in Fig. 2. | ||
| Fig. 2 Structures of RAFT agents synthesized: O-ethyl-S-(1-methoxycarbonyl) ethyl-dithiocarbonate (X1), alkyne-terminated RAFT agent (AT-X1) and protected alkyne-terminated RAFT agent (PAT-X1). | ||
X1 was synthesized through the reaction of potassium ethyl xanthogenate with methyl 2-bromopropionate in methanol according to the similar procedures reported in literature for analogous RAFT agents.51,52 The success of the reactions was confirmed by 1H NMR (ESI, Fig. S1†). Although the impurities after the synthesis are very small, the purification procedures are crucial to avoid RAFT agent contaminations that could interfere with the success of polymerization.
The synthesis of the AT-X1 was already been reported by Patel and co-authors for the polymerization of N-vinylpyrrolidone.48
However, the method reported here is easier, cleaner and more efficient than the aforementioned method that involves the carbodiimide activation. The synthesis of AT-X1 was carried out into two steps; firstly, the synthesis of the alkyne terminated bromide through the reaction of 2-bromopropionyl chloride and propargyl alcohol (PA), followed by the bromine substitution with the potassium ethyl xanthogenate (Fig. 3). The success of the reaction was confirmed by 1H and 13C NMR spectroscopy (Fig. 4, S2 and S3 (ESI)†). The FTIR-ATR spectra of the AT-X1 (Fig. S4, ESI†) shows the presence of the characteristic alkyne C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) H stretch vibration at 3300 cm−1,47 and also –C
H stretch vibration at 3300 cm−1,47 and also –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and C–S stretching vibrations at 1044 cm−1 and 633 cm−1, respectively.
S and C–S stretching vibrations at 1044 cm−1 and 633 cm−1, respectively.
It is known that the alkyne group in the RAFT agent may interfere with the radical polymerization process.45 In order to evaluate this possibility, the synthesis of new protected RAFT agent (protected alkyne-terminated RAFT agent (PAT-X1)) using a trimethyl silyl group was envisaged. For the synthesis of the PAT-X1, 2-(ethoxycarbonothioylthio)propanoic acid, was firstly synthesized through the reaction of potassium ethyl xanthogenate and 2-bromopropionic acid in methanol. The further coupling reaction with 5-trimethylsilyl-4-pentyn-1-ol originates PAT-X1 (Fig. 5). The success of the reaction was confirmed by 1H NMR (Fig. 6) and 13C NMR (ESI Fig. S5†) (‡53–55).
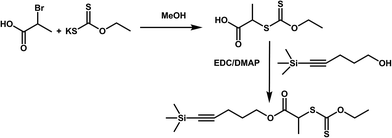 | ||
| Fig. 5 Schematic representation of the synthesis strategy of protected alkyne-terminated RAFT agent (PAT-X1). | ||
Polymerization of VAc using the synthesized RAFT agents
To investigate the ability of the RAFT agents to control the polymerization of non-activated monomers, VAc was used as a model monomer. The polymerization of VAc was carried out using the different xanthates in 1,4-dioxane ([1,4-dioxane]0/[VAc]0 = 1/1 (w/w)) at 60 °C and initiated by the AIBN. The ratio of RAFT agent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AIBN was kept at 1
AIBN was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2. Monomer conversions were calculated using 1H NMR spectroscopy by comparing the integrations of the –CH signals on the VAc (δ = 4.50 ppm) with the corresponding signals of the polymer backbone (δ = 4.80). X1 was used for comparison purposes, since it was already reported for the synthesis of well-defined PVAc.34–36 Despite the good results obtained when X1 was used (Fig. 7), the polymer does not have the necessary functionality that would allow further coupling reactions with other molecules or polymers. In the case of X1, polymer post-modification reactions would be required in order to achieve a specific functionality in the polymer chain-end. In this context, the concept of the direct introduction of the desired functionality in the RAFT agent is preferable.
0.2. Monomer conversions were calculated using 1H NMR spectroscopy by comparing the integrations of the –CH signals on the VAc (δ = 4.50 ppm) with the corresponding signals of the polymer backbone (δ = 4.80). X1 was used for comparison purposes, since it was already reported for the synthesis of well-defined PVAc.34–36 Despite the good results obtained when X1 was used (Fig. 7), the polymer does not have the necessary functionality that would allow further coupling reactions with other molecules or polymers. In the case of X1, polymer post-modification reactions would be required in order to achieve a specific functionality in the polymer chain-end. In this context, the concept of the direct introduction of the desired functionality in the RAFT agent is preferable.
The kinetic data presented in Fig. 7 for the RAFT agent X1 reveals that the polymer Mn increases linearly with monomer conversion and Đ remain below 1.4 throughout the polymerization. The obtained values are in accordance with similar literature reports for xanthate mediated RAFT polymerizations of Vac in bulk32 or in ethyl acetate.35,36
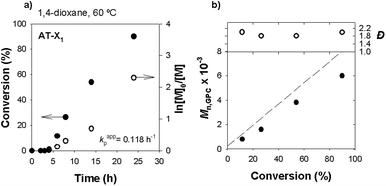
The AT-X1 and PAT-X1 are xanthate molecules with similar structure to X1 but with a slight variation of the end-group functionality of the leaving group (R group). The protection of the alkyne moiety (PAT-X1) was performed in order to observe the role of the terminal alkyne in the polymerization course. It is known that the alkyne hydrogen in the end of the leaving group of the RAFT agent may interfere with the radical polymerization.45 In fact, when X1 was replaced by AT-X1, using similar reaction conditions, the homopolymerization of VAc was slower, and larger Đ were observed, as shown in the kinetic data presented in Fig. 8 and GPC traces in Fig. S6 (ESI†).
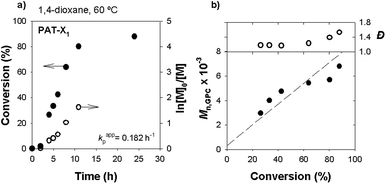
Fig. 9 presents the kinetic data obtained for the homopolymerization of VAc using the protected-alkyne RAFT agent. The first-order kinetic and the linear evolution of the MW with the conversion indicate a controlled polymerization. It is interesting to notice that below 70% conversion, the Đ values are very low for a non-activated monomer such as VA (Đ < 1.2), but tend to increase for high monomer conversions. For conversions above 80%, the Mn,GPC values become smaller than Mn,th. This observation may be ascribed to irreversible transfer reactions, chain transfer reactions to monomer and polymer, due to the very reactive nature of the VAc propagating radical.56 This effect is mostly detected in the polymer GPC traces by the presence of a prominent low MW tail (high retention volume) (Fig. 10) and consequent broad MW distributions from moderate to higher monomer conversions. Similar results were already reported for similar RAFT polymerization of VAc (bulk, 60 °C).57,58 It should be noted the presence of an induction period for the different RAFT agents studied, which could be related with slower reinitiation of the initial RAFT agent.59 Moreover, several literature reports dealing with the xanthate mediated RAFT polymerization of PVAc reported a similar observation.60,61 Even so, for our novel PAT-X1, the observed induction period is much smaller. In all cases, the molar mass evolution plots show a discrepancy between the values of Mn,th and Mn,GPC, increasing with conversion. This result may be related with the high concentration of initiator used, which consequently increases the concentration of radicals in the system and promotes irreversible termination reactions. The summary of all experiments performed using the three different RAFT agents is shown in Table 1. The results suggest that only the RAFT agents X1 and PAT-X1 were able to conduct a controlled polymerization of VAc with relatively narrow MW distributions, up to high conversions. Despite the high Đ value obtained for the last kinetic point using PAT-X1, in comparison with the X1, the kinetic results present in Fig. 9 and 10, indicate that the Đ values tend to increase with reaction conversion. The broad MW distribution observed for the reactions with the alkyne functionalized RAFT agent (AT-X1) prove the inefficiency of such compound to conduct a controlled polymerization of VAc. On this matter, the Đ values of the PVAc synthesized through RAFT using AT-X1 or using FRP conditions are similar (Đ = 2.2).
| Entry | RAFT agent | Initiator | Temp., °C | Timea ,h | Conv.a, % | k appp, h−1 | M n,th a × 103 | M n,GPC a × 103 | Đ a |
|---|---|---|---|---|---|---|---|---|---|
| a Values obtained from the last sample from the kinetic study. | |||||||||
| 1 | X1 | AIBN | 60 | 24 | 98 | 0.291 | 8.7 | 6.80 | 1.4 |
| 2 | AT-X1 | AIBN | 60 | 24 | 90 | 0.118 | 8.0 | 6.0 | 2.0 |
| 3 | PAT-X1 | AIBN | 60 | 32 | 96 | 0.182 | 8.52 | 6.69 | 1.6 |
| 4 | — | AIBN | 60 | 16 | — | — | — | 6.06 | 2.2 |
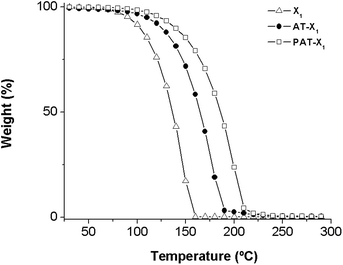
Thermogravimetric analysis of the RAFT agents
The thermal behavior of RAFT agents was analyzed by TGA (Fig. 11). The results reveal a single step of mass loss, at relatively low temperatures, for the different RAFT agents synthesized. In order to evaluate the nature of this event, a preparative experiment in a sealed flask under nitrogen was carried out, at 130 °C during 2 h. The comparison of 1H NMR spectra of the RAFT agent before and after the thermal treatment has shown no differences on the peak integrations, which indicates that the mass loss observed in TGA traces are related to volatilization. No significant mass loss was observed after the experiment. Regarding the effect of the structure of R group, the results suggest that the volatilization temperature increases with the following order: PAT-X1 > AT-X1 > X1.PVAc chain-end functionality
The chemical structure of the PVAc synthesized by RAFT polymerization was determined with 1H NMR, FTIR-ATR and UV-Vis analysis. Fig. 12 shows the 1H NMR spectrum of a PVAc sample synthesized using the PAT-X1. The characteristic peaks of the PVAc structure at 1.75 ppm (o, o′, –CH2–CH–), 2.02 ppm (n, n′, –CH3), 4.86 ppm (m, –CH2–CH–) and 6.62 ppm (m′, –CH2–CH–S) are in agreement with the data reported in the literature.37 The retention of RAFT functionality is evidenced by the peaks from the PAT-X1 at 0.14 ppm (k, –Si(CH3)3–), 0.88 ppm (a, –CH2–CH3), 1.17 ppm (d, –CH–CH3), 2.31 ppm (j, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH2), 3.74 ppm (b, –CH2–CH3), 4.07 ppm (c, –CH–CH3) and 4.15 ppm (h, –CH2–O–). It should be noted that the ratio between the integral of k and a signals does not correspond to the theoretical value assuming the number of protons from the R and Z groups, respectively. This result may be justified by the unavoidable termination reactions that occur in any radical based polymerization method. It should not be excluded also a possible loss of RAFT chain end functionality during the purification steps.
C–CH2), 3.74 ppm (b, –CH2–CH3), 4.07 ppm (c, –CH–CH3) and 4.15 ppm (h, –CH2–O–). It should be noted that the ratio between the integral of k and a signals does not correspond to the theoretical value assuming the number of protons from the R and Z groups, respectively. This result may be justified by the unavoidable termination reactions that occur in any radical based polymerization method. It should not be excluded also a possible loss of RAFT chain end functionality during the purification steps.
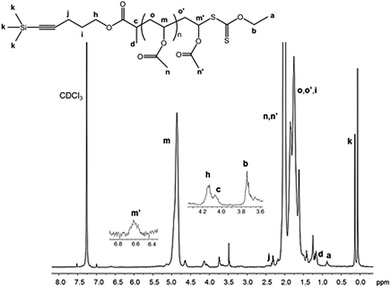 | ||
| Fig. 12 The 1H NMR spectrum of PVAc synthesized by RAFT using PAT-X1 (Mn,GPC = 6.0 × 103; Mn,NMR = 7.56 × 103, Đ = 1.36) in CDCl3. | ||
The preservation of the terminal chains ends during the RAFT reaction can also be accessed by FTIR-ATR analysis. The FTIR-ATR spectra of the PAT-X1 and the PVAc synthesized using the PAT-X1 is shown in Fig. 13. The bands at 2850–2940 cm−1 are associated to both symmetric and asymmetric C–H stretching vibrations. A strong absorption band at 1740 cm−1 is related with the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration (carbonyl bond of the ester). The characteristic bands of the xanthate group, –C
O stretching vibration (carbonyl bond of the ester). The characteristic bands of the xanthate group, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and C–S, at 1044 cm−1 and 633 cm−1 respectively and the characteristic bands of the trimethylsilyl group (–Si–(CH3)3), at 755 cm−1 and 840 cm−1 are present in both FTIR-ATR spectra. Ultraviolet-Visible (UV-Vis) spectroscopy has been used as an efficient tool to identify the presence of the characteristic –C
S and C–S, at 1044 cm−1 and 633 cm−1 respectively and the characteristic bands of the trimethylsilyl group (–Si–(CH3)3), at 755 cm−1 and 840 cm−1 are present in both FTIR-ATR spectra. Ultraviolet-Visible (UV-Vis) spectroscopy has been used as an efficient tool to identify the presence of the characteristic –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S end-group of polymers prepared by RAFT polymerization. The polymer Z-group can be lost during the polymerization process due to side reactions of the thiocarbonyl groups or due to the inherent termination reactions that occurs during the polymerization.62 The UV-Vis spectra in CHCl3 of all synthesised PVAc are presented in ESI (Fig. S7†). All samples show an absorption band below 300 nm ascribed to the thiocarbonyl bound, indicating the presence of such groups in the final polymer backbone. This thiocarbonyl group can be further removed or transformed in order to achieve a desired functionality and easily conjugate the polymer with other molecules or polymer segments.63,64
S end-group of polymers prepared by RAFT polymerization. The polymer Z-group can be lost during the polymerization process due to side reactions of the thiocarbonyl groups or due to the inherent termination reactions that occurs during the polymerization.62 The UV-Vis spectra in CHCl3 of all synthesised PVAc are presented in ESI (Fig. S7†). All samples show an absorption band below 300 nm ascribed to the thiocarbonyl bound, indicating the presence of such groups in the final polymer backbone. This thiocarbonyl group can be further removed or transformed in order to achieve a desired functionality and easily conjugate the polymer with other molecules or polymer segments.63,64
Chain extension reaction
The PVAc synthesized through RAFT polymerization (Mn,GPC = 3.01 × 103, Đ = 1.20) was purified and used as macro-RAFT agent. Fig. 14 shows the complete shift of the molecular weight distribution from a PVAc macroinitiator (macro PAT–PVAc) to a higher MW values (extended PVAc, Mn,GPC = 10.64 × 103, Đ = 1.88) confirming the “living” nature of the polymer. | ||
| Fig. 14 The GPC traces of PAT–PVAc samples before (on the right) and after chain extension (on the left) experiment. | ||
Deprotection of PVAc and coupling reaction with N3-PEG
After the RAFT polymerization of VAc using the PAT-X1, the protective trimethyl silyl group was removed using TBAF (Fig. 15 (1.2)). The success of the reaction was confirmed by the disappearance of the characteristic trimethyl silyl signal (k) at 0.14 ppm by the 1H NMR spectrum of the unprotected PVAc (Fig. 12 (k) and S8, ESI†).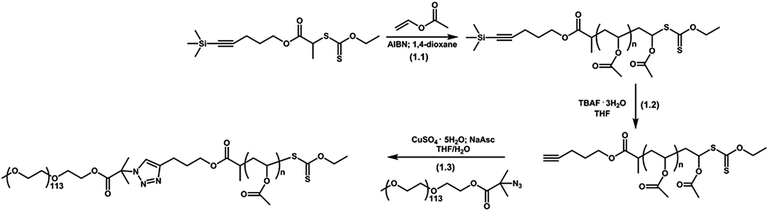 | ||
| Fig. 15 Schematic representation of the RAFT polymerization of VAc (1.1), PVAc deprotection (1.2) and synthesis of PEG-b-PVAc block copolymers by CuAAC reaction (1.3). | ||
As a proof of concept an azide terminated PEG (N3-PEG) was used for the post-polymerization coupling reaction with an alkyne-terminated PVAc (Fig. 15 (1.3)). In fact, this reaction could not be named as “click” reaction since it did not fulfill all of the criteria in the context of macromolecular chemistry.65 The success of the synthesis of the PEG-b-PVAc copolymer was evaluated by GPC and FTIR-ATR spectroscopy. Fig. 16 shows the clear shift of the molecular weight distribution of the PVAc block towards higher molecular weight values. It should be stressed the presence of a shoulder that based on the GPC traces can be ascribed to the some unreacted PVAc segments. Moreover, the comparison of FTIR-ATR spectra of the homopolymer precursors, N3-PEG and AT-PVAc with the copolymer PEG-b-PVAc (Fig. S9, ESI†), confirms the presence of the characteristic bands from each homopolymer segment in the spectrum of the copolymer and reveals the disappearance of the characteristic azide signal at 2100 cm−1, confirming the success of the coupling reaction.
 | ||
| Fig. 16 GPC traces of the RALS signal of the PVAc and PVAc-b-PEG copolymer, after the coupling reaction. | ||
Conclusions
We reported the synthesis of one protected alkyne containing xanthate RAFT agent, able to efficiently control the polymerization of VAc. The protection of the alkyne moiety in the RAFT agent is crucial to afford a polymerization with a good control over the MW and with low Đ. The structural analysis of the PVAc, performed by 1H NMR, FTIR-ATR and UV-Vis experiments reveal the retention of the chain-end functionality. The “living” nature of the PVAc synthesized through RAFT was confirmed by a successful chain extension experiment. After the deprotection of the alkyne end group, the well-defined alkyne-terminated PVAc can be easily conjugated with azido-terminated structures through a CuAAC reaction.Acknowledgements
Joana R. Góis acknowledges FCT-MCTES for her PhD scholarship (SFRH/BD/69635/2010). The NMR data was collected at the UC-NMR facility which is supported in part by FEDER – European Regional Development Fund through the COMPETE Programme (Operational Programme for Competitiveness) and by National Funds through FCT – Fundação para a Ciência e a Tecnologia (Portuguese Foundation for Science and Technology) through grants REEQ/481/QUI/2006, RECI/QEQ-QFI/0168/2012, CENTRO-07-CT62-FEDER-002012, and Rede Nacional de Ressonância Magnética Nuclear (RNRMN).Notes and references
- P. J. Roth, C. Boyer, A. B. Lowe and T. P. Davis, Macromol. Rapid Commun., 2011, 32, 1123–1143 CrossRef CAS PubMed.
- A. Ghadban and L. Albertin, Polymers, 2013, 5, 431–526 CrossRef PubMed , p. 496.
- D. Konkolewicz, Y. Wang, M. Zhong, P. Krys, A. A. Isse, A. Gennaro and K. Matyjaszewski, Macromolecules, 2013, 46, 8749–8772 CrossRef CAS.
- C.-H. Peng, T.-Y. Yang, Y. Zhao and X. Fu, Org. Biomol. Chem., 2014, 12, 8580–8587 CAS.
- W. Wang, J. Zhao, N. Zhou, J. Zhu, W. Zhang, X. Pan, Z. Zhang and X. Zhu, Polym. Chem., 2014, 5, 3533–3546 RSC.
- J. P. Mendes, F. Branco, C. M. R. Abreu, P. V. Mendonca, A. V. Popov, T. Guliashvili, A. C. Serra and J. F. J. Coelho, ACS Macro Lett., 2014, 3, 544–547 CrossRef CAS PubMed.
- C. M. R. Abreu, A. C. Serra, A. V. Popov, K. Matyjaszewski, T. Guliashvili and J. F. J. Coelho, Polym. Chem., 2013, 4, 5629–5636 RSC.
- J. R. Gois, D. Konkolewic, A. V. Popov, T. Guliashvili, K. Matyjaszewski, A. C. Serra and J. F. J. Coelho, Polym. Chem., 2014, 5, 4617–4626 RSC.
- T. Guliashvili, P. V. Mendonca, A. C. Serra, A. V. Popov and J. F. J. Coelho, Chem.–Eur. J., 2012, 18, 4607–4612 CrossRef CAS PubMed.
- P. V. Mendonca, A. C. Serra, J. F. J. Coelho, A. V. Popov and T. Guliashvili, Eur. Polym. J., 2011, 47, 1460–1466 CrossRef CAS PubMed.
- V. Percec, A. V. Popov, E. Ramirez-Castillo, J. F. J. Coelho and L. A. Hinojosa-Falcon, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 6267–6282 CrossRef CAS PubMed.
- C. M. R. Abreu, P. V. Mendonça, A. C. Serra, J. F. J. Coelho, A. V. Popov, G. Gryn’ova, M. L. Coote and T. Guliashvili, Macromolecules, 2012, 45, 2200–2208 CrossRef CAS.
- Y. Piette, A. Debuigne, C. Jerome, V. Bodart, R. Poli and C. Detrembleur, Polym. Chem., 2012, 3, 2880–2891 RSC.
- I. J. Johnson, E. Khosravi, O. M. Musa, R. E. Simnett and A. M. Eissa, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 775–786 CrossRef CAS PubMed.
- M. C. Iovu and K. Matyjaszewski, Macromolecules, 2003, 36, 9346–9354 CrossRef CAS.
- V. Percec, T. Guliashvili, J. S. Ladislaw, A. Wistrand, A. Stjerndahl, M. J. Sienkowska, M. J. Monteiro and S. Sahoo, J. Am. Chem. Soc., 2006, 128, 14156–14165 CrossRef CAS PubMed.
- S. Harrisson, X. Liu, J.-N. Ollagnier, O. Coutelier, J.-D. Marty and M. Destarac, Polymers, 2014, 6, 1437 CrossRef CAS PubMed.
- J. P. Mendes, F. Branco, C. M. R. Abreu, P. V. Mendonça, A. C. Serra, A. V. Popov, T. Guliashvili and J. F. J. Coelho, ACS Macro Lett., 2014, 3, 858–861 CrossRef CAS.
- T. Huadong, R. Maciej and S. Youqing, in Controlled/Living Radical Polymerization: Progress in ATRP, American Chemical Society, 2009, vol. 1023, ch. 10, pp. 139–157 Search PubMed.
- V. Percec, A. V. Popov, E. Ramirez-Castillo and J. F. J. Coelho, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 773–778 CrossRef CAS PubMed.
- D. Braun, J. Vinyl Addit. Technol., 2005, 11, 86–90 CrossRef CAS PubMed.
- C.-H. Peng, J. Scricco, S. Li, M. Fryd and B. B. Wayland, Macromolecules, 2008, 41, 2368–2373 CrossRef CAS.
- Y.-C. Lin, Y.-L. Hsieh, Y.-D. Lin and C.-H. Peng, Macromolecules, 2014, 47, 7362–7369 CrossRef CAS.
- A. Kermagoret, Y. Nakamura, M. Bourguignon, C. Detrembleur, C. Jérôme, S. Yamago and A. Debuigne, ACS Macro Lett., 2014, 3, 114–118 CrossRef CAS.
- A. N. Morin, C. Detrembleur, C. Jérôme, P. de Tullio, R. Poli and A. Debuigne, Macromolecules, 2013, 46, 4303–4312 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379–410 CrossRef CAS.
- G. Paradossi, F. Cavalieri, E. Chiessi, C. Spagnoli and M. Cowman, J. Mater. Sci.: Mater. Med., 2003, 14, 687–691 CrossRef CAS.
- M. Destarac, Polym. Rev., 2011, 51, 163–187 CrossRef CAS PubMed.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2012, 65, 985–1076 CrossRef CAS.
- C. Boyer, V. Bulmus, T. P. Davis, V. Ladmiral, J. Q. Liu and S. Perrier, Chem. Rev., 2009, 109, 5402–5436 CrossRef CAS PubMed.
- P. E. Dufils, G. David, B. Boutevin, G. Woodward, G. Otter, A. Guinaudeau, S. Mazières and M. Destarac, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1997–2007 CrossRef CAS PubMed.
- M. H. Stenzel, L. Cummins, G. E. Roberts, T. P. Davis, P. Vana and C. Barner-Kowollik, Macromol. Chem. Phys., 2003, 204, 1160–1168 CrossRef CAS PubMed.
- R. W. Simms, T. P. Davis and M. F. Cunningham, Macromol. Rapid Commun., 2005, 26, 592–596 CrossRef CAS PubMed.
- A. Theis, T. P. Davis, M. H. Stenzel and C. Barner-Kowollik, Polymer, 2006, 47, 999–1010 CrossRef CAS PubMed.
- J. Schmitt, N. Blanchard and J. Poly, Polym. Chem., 2011, 2, 2231–2238 RSC.
- E. Girard, T. Tassaing, J.-D. Marty and M. Destarac, Polym. Chem., 2011, 2, 2222–2230 RSC.
- V. K. Patel, N. K. Vishwakarma, A. K. Mishra, C. S. Biswas and B. Ray, J. Appl. Polym. Sci., 2012, 125, 2946–2955 CrossRef CAS PubMed.
- D. Charmot, P. Corpart, H. Adam, S. Z. Zard, T. Biadatti and G. Bouhadir, Macromol. Symp., 2000, 150, 23–32 CrossRef CAS.
- D. J. Keddie, C. Guerrero-Sanchez, G. Moad, R. J. Mulder, E. Rizzardo and S. H. Thang, Macromolecules, 2012, 45, 4205–4215 CrossRef CAS.
- M. Destarac, D. Charmot, X. Franck and S. Z. Zard, Macromol. Rapid Commun., 2000, 21, 1035–1039 CrossRef CAS.
- D. J. Keddie, Chem. Soc. Rev., 2014, 43, 496–505 RSC.
- D. Fournier, R. Hoogenboom and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 1369–1380 RSC.
- M. Benaglia, J. Chiefari, Y. K. Chong, G. Moad, E. Rizzardo and S. H. Thang, J. Am. Chem. Soc., 2009, 131, 6914–6915 CrossRef CAS PubMed.
- A. Gregory and M. H. Stenzel, Prog. Polym. Sci., 2012, 37, 38–105 CrossRef CAS PubMed.
- D. Quemener, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Chem. Commun., 2006, 5051–5053 RSC.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- D. Quémener, M. L. Hellaye, C. Bissett, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 155–173 CrossRef PubMed.
- V. K. Patel, N. K. Vishwakarma, A. K. Mishra, C. S. Biswas, P. Maiti and B. Ray, J. Appl. Polym. Sci., 2013, 127, 4305–4317 CrossRef CAS PubMed.
- X. Sun, H. Zhang, X. Huang, X. Wang and Q.-F. Zhou, Polymer, 2005, 46, 5251–5257 CrossRef CAS PubMed.
- N. Rocha, P. V. Mendonca, J. P. Mendes, P. N. Simoes, A. V. Popov, T. Guliashvili, A. C. Serra and J. F. J. Coelho, Macromol. Chem. Phys., 2013, 214, 76–84 CrossRef CAS PubMed.
- M. Destarac, C. Brochon, J.-M. Catala, A. Wilczewska and S. Z. Zard, Macromol. Chem. Phys., 2002, 203, 2281–2289 CrossRef CAS PubMed.
- H. Mori, E. Kudo, Y. Saito, A. Onuma and M. Morishima, Macromolecules, 2010, 43, 7021–7032 CrossRef CAS.
- E. L. Eliel, Top. Curr. Chem., 1982, 105, 1–76 CrossRef CAS.
- S. Fujita, Tetrahedron, 2006, 62, 691–705 CrossRef CAS PubMed.
- R. Bentley, Chem. Educ., 2007, 12, 316–321 CAS.
- D. Britton, F. Heatley and P. A. Lovell, Macromolecules, 1998, 31, 2828–2837 CrossRef CAS.
- J. Bernard, A. Favier, L. Zhang, A. Nilasaroya, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Macromolecules, 2005, 38, 5475–5484 CrossRef CAS.
- A. Favier, C. Barner-Kowollik, T. P. Davis and M. H. Stenzel, Macromol. Chem. Phys., 2004, 205, 925–936 CrossRef CAS PubMed.
- G. Pound, J. B. McLeary, J. M. McKenzie, R. F. M. Lange and B. Klumperman, Macromolecules, 2006, 39, 7796–7797 CrossRef CAS.
- Y. Yan, W. Zhang, Y. Qiu, Z. Zhang, J. Zhu, Z. Cheng, W. Zhang and X. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5206–5214 CrossRef CAS PubMed.
- R. Fleet, J. B. McLeary, V. Grumel, W. G. Weber, H. Matahwa and R. D. Sanderson, Macromol. Symp., 2007, 255, 8–19 CrossRef CAS PubMed.
- K. Skrabania, A. Miasnikova, A. M. Bivigou-Koumba, D. Zehm and A. Laschewsky, Polym. Chem., 2011, 2, 2074–2083 RSC.
- H. Willcock and R. K. O'Reilly, Polym. Chem., 2010, 1, 149–157 RSC.
- D. J. Keddie, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 2012, 45, 5321–5342 CrossRef CAS.
- C. Barner-Kowollik, F. E. Du Prez, P. Espeel, C. J. Hawker, T. Junkers, H. Schlaad and W. van Camp, Angew. Chem., Int. Ed., 2011, 50, 60–62 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Methods and characterization details. See DOI: 10.1039/c5ra15580k |
‡ It should be noted that all three target molecules contain a chiral center C*HMeS(CO). It makes geminal methylene protons CHH diastereotopic and causes anisochrony due to unequal magnetic field sensed by these nuclei. The resonance signals of these protons are overlapped in the spectrum of PAT-X1, whereas the 1H NMR spectra of X1 and AT-X1 revealed resolved peaks of each such proton. Therefore, the difference in chemical shifts for these anisochronic protons is 0.0135 ppm for –OCHHCH3 in X1, 0.0014 ppm for CH![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CHHO– and 0.0009 ppm for –OCHHCH3 in AT-X1. Interestingly, the chemical shift difference of 0.0075 ppm for CH C–CHHO– and 0.0009 ppm for –OCHHCH3 in AT-X1. Interestingly, the chemical shift difference of 0.0075 ppm for CH![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CHHO– diastereotopic protons in propargyl 2-bromopropionate is almost equal to 4JHH coupling constant, thus making a quasitriplet from the two doublets (ESI). C–CHHO– diastereotopic protons in propargyl 2-bromopropionate is almost equal to 4JHH coupling constant, thus making a quasitriplet from the two doublets (ESI). |
| This journal is © The Royal Society of Chemistry 2015 |