Direct growth of flower-like 3D MnO2 ultrathin nanosheets on carbon paper as efficient cathode catalyst for rechargeable Li–O2 batteries†
Hong-Qiang Wangac,
Jing Chena,
Si-Jiang Huc,
Xiao-Hui Zhanga,
Xiao-Ping Fana,
Juan Dud,
You-Guo Huanga and
Qing-Yu Li*b
aSchool of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Guilin 541004, China. E-mail: liqingyu62@126.com; Fax: +86 0773 5858562
bGuangxi Key Laboratory of Low Carbon Energy Materials, School of Chemical and Pharmaceutical Sciences, Guangxi Normal University, Guilin 541004, China
cHubei Key Laboratory for Processing and Application of Catalytic Materials, Huanggang Normal University, Huanggang 438000, China
dCentral South University School of Metallurgy and Environment, Central South University, Changsha 410083, China
First published on 19th August 2015
Abstract
Flower-like 3D MnO2 ultrathin nanosheets were synthesized by direct growth of MnO2 on carbon paper (CP) through a facile electro-deposition method. When applied as a self-supporting, binder-free cathode material for rechargeable non-aqueous lithium-oxygen batteries, the as-prepared MnO2/CP electrode exhibited comparable catalytic performance (discharge capacity of 5132 mA h g−1 at a current density of 500 mA g−1) and long cycle stability (up to 125 cycles with controlling capacity of 500 mA h g−1). The superior performance is proposed to be associated with the 3D nanoporous structures and abundant oxygen defects as well as the absence of side reactions related to carbon based conductive additives and binders.
Nonaqueous lithium–oxygen batteries [LOBs] have a much superior theoretical gravimetric energy density compared to conventional lithium-ion batteries.1–4 A typical rechargeable non-aqueous LOBs theoretically exhibits a high energy density of 3552 W h kg−1 that is even compatible with that of gasoline.5 Electrocatalysts in the cathode play a key role in both oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) of LOBs, have significant influence on the power density, cyclic ability and energy efficiency of batteries.6–8 Up to now, a variety of catalytic materials including noble metals and alloys,9–12 carbon materials,13–15 and transition metal oxides/nitride16–20 have been studied to address the sluggish kinetics of the cathode reactions. Among those, transition metal oxides in particular manganese dioxide (MnO2) have been widely investigated because of their high intrinsic activity, low cost, abundance, and high catalytic activity toward ORR in alkaline media.21,22
However, the intrinsically low electronic conductivity of manganese oxides generally limit their electrochemical performance. To enhance the electron conduction, manganese oxides are commonly supported on or mixed with conductive agents.23–25 Another strategy is growing metal oxide directly on electrically conductive substrates such as carbon paper, Ni or other porous substrate.26–28 Due to the considerable chemical stability and electronic conductivity, carbon paper has been widely used in batteries as both the support and the current collector.29 The in situ preparation of catalysts directly growth onto porous substrates without the need of additional (inactive) binders or conductive additives is a way to increasing catalyst efficacy and utility. Direct contact with the porous substrate collector promotes mechanical adhesion and facile interfacial electron transfer between the current collector and the catalyst.30 There are a lot of in situ synthesis method such as hydrothermal method, chemical deposition method, electro-deposition method and so on. The electro-deposition method is a convenient and practical method by which a uniform deposition on a conductive substrate can be achieved at room temperature.31
Herein, we report a new design of binder-free air electrode constructed by direct growth of MnO2 on CP through a facile electro-deposition method.32,33 As shown in Fig. 1. Compared with conventional paste coating method, this method makes it possible to deposit the catalyst only on the skeleton of CP with the intrinsic pores of unblocked, in favor of free oxygen transport and easy electrolyte penetration.26 Another advantage of the air electrode is the MnO2, unlike other MnO2 polymorphs, which is generally crystallized into ultrathin nanosheets, then self-assemble into porous flower-like 3D morphologies during the electro-deposition process.34,35 Recently, the flower like MnO2 has been used in LOBs achieving high discharge capacity of 4581 mA h g−1.42 The combination of flower-like MnO2 nanostructure and CP is advantageous to the transport of electrons, oxygen, and Li+. Based on these advantages, the hybrid of MnO2/CFP shows good performance and application prospect.
The microstructures of the CP and MnO2/CP hybrid were examined using scanning electron microscopy (SEM). A porous CP, with an interconnected 3D scaffold (Fig. 2a), was used as a template for the growth of MnO2. The MnO2 was coated on the surface of the carbon fiber by electro-deposition method. The images indicate that the MnO2 nanosheets were clearly formed on the carbon fiber and the CP skeleton is almost fully and uniformly covered by the MnO2 nanosheets (Fig. 2b and c). The optical image in Fig. 2d reveals that the flower-like MnO2 has a self-supported structure around 200–500 nm in diameter constructed with intersected ultrathin nanosheets. The MnO2 ultrathin nanosheets self-assemble into porous flower-like 3D morphologies without any other binders. The SEM images show typical interconnected, ultrathin nanosheets, and flower-like MnO2 particles grown on the surface of the CP, while the porous structure and pore size of CP are kept. Due to the shortest pathway in the through-thickness direction binder-free electrode can reduce the electrical resistance, expose all active surface area to support the electrochemical process and exclude all the possible side-effects associate with the binder decomposition.
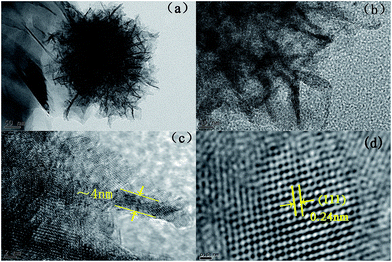
In order to further investigate the detailed morphology and microstructure of the MnO2 ultrathin nanosheets of the MnO2/CP electrode, the materials exfoliated from the electrode were characterized by transmission electron microscopy (TEM), as shown in Fig. 3. TEM image in Fig. 3a indicates that the flower-like material is an assembly of thin nanosheets, agreeing well with the SEM observation. The flower-like material was stick to the carbon fiber indicate good adhesive power of the MnO2 synthesis by electro-deposition. The nanosheets tend to cross together form a three-dimensional network structure and nanopore (Fig. 3b), which allows O2 and electrolyte easy to get the surface of nanosheets. The typical thickness of the nanosheet is measured to be around 4 nm (Fig. 3c), corresponding to about 5–8 layered MnO2 crystal unit cells. A high-resolution TEM image (Fig. 3d) shows lattice fringes distance of 0.24 nm, which agrees with the spacing of the (111) plane of birnessite-type MnO2 (JCPDS 18-0802).
The flower-like 3D MnO2 ultrathin nanosheets synthesized here by control the current density resulting in a highly 3D porous structure that promotes efficient contacts between the active material and the electrolyte, providing more active sites for electrochemical reactions. The absence of binder supply additional accessible space for MnO2. Moreover, the flower-like 3D structure can provide short diffusion path lengths to both ions and electrons and also sufficient porosity for electrolyte penetration giving rise to high charging and discharging rates.36,37
To investigate the chemical states of the MnO2/CP composite, X-ray photoelectron spectroscopy (XPS) studies were performed. Fig. 4a shows the XPS survey scan of the sample. We can see a 1.56 wt% percentage composition of Mn. Fig. 4b–d exhibits the Mn2p, C1s and O1s peaks, which were analyzed by the software of XPS peak version 4.1, as shown in Fig. 4b, the Mn in the nanocomposites of MnO2/CP is composed of various surface chemical states, including metallic Mn4+, Mn3+ and Mn2+.38 The main Mn2p3/2 peak located at 642.2 eV and can be assigned to Mn4+ from MnO2,39 indicating that the Mn element mainly existed as a chemical state of Mn4+. The peaks located at 642.8 and 641.5 eV, which are characteristic of the Mn3+ and Mn2+, respectively.39,40 In the C1s spectra of Fig. 4c, there are three peaks located at 284.5, 284.6 and 285.3 eV are related to C1s binding energy in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C and C–H respectively.41 And in the O1s spectra of Fig. 4d, the three peaks located at 529.8, 531.4 and 532.3 eV are related to O1s binding energy in anhydrous (Mn–O–Mn), hydrated (Mn–O–H) manganese oxides and hydrated (H–O–H), respectively.43
C, C–C and C–H respectively.41 And in the O1s spectra of Fig. 4d, the three peaks located at 529.8, 531.4 and 532.3 eV are related to O1s binding energy in anhydrous (Mn–O–Mn), hydrated (Mn–O–H) manganese oxides and hydrated (H–O–H), respectively.43
 | ||
| Fig. 4 XPS spectra of the MnO2/CP composite. (a) Survey scan. (b) Mn2p spectrum. (c) C1s spectrum. (d) O1s spectrum. | ||
The Brunauer–Emmett–Teller (BET) surface areas of the CP and MnO2/CP electrodes were measured by nitrogen adsorption–desorption isotherms, as shown in Fig. 5. Results show that the CP and MnO2/CP electrodes of BET specific surface areas are 2.68 m2 g−1 and 4.72 m2 g−1 respectively. The BET specific surface area of CP shows that the conductive substrates has a relatively high specific surface areas than other substrates. The BET specific surface area of MnO2/CP is about 2 times of the pristine CP, which shows the high BET specific surface area of flower-like MnO2. The large surface area of MnO2 supplies a high concentration of catalytic sites and its porous structure provides sufficient voids for Li2O2 deposition.
The cycling stability of Li–O2 battery was investigated to evaluate the long-term catalytic activity of the MnO2/CP electrode. As shown in Fig. 6a and b, the Li–O2 battery was cycled under 100 mA g−1 at 500 mA h g−1 based on mass of MnO2 over a wide voltage window (2.0–5.0 V). Interestingly, the battery with MnO2/CP electrode can sustain 125 cycles and most of the discharge capacities are delivered above 2.5 V and most of charge capacities are delivered below 4.5 V. The full capacity discharge–charge cycle performance of the LOBs was examined at the current density of 500 mA g−1 over a wide voltage window (2–4.5 V). As shown in Fig. 6c and d, the MnO2/CP electrode delivered a reasonable high capacity of 5136 mA h g−1 at the first discharge process and keeps about 1000 mA h g−1 for 20 cycles. Moreover, the charge capacity is close to the discharge capacity at each cycle with a coulombic efficiency of approximately 100%. The above results should be attributed to the excellent catalytic activity and unique porous structure of MnO2, which benefit the formation and decomposition of Li2O2 within the pores of the flower-like MnO2.
In spite of high discharge capacity (5136 mA h g−1) at first cycle under 500 mA g−1, the capacity decay quickly to about 2900 mA h g−1 at the 5th discharge process. EIS and SEM at different discharge–charge stages was measured to get insight into the electrochemical performance of the 3D MnO2/CP electrode (Fig. 7 and 8). As shown in Fig. 8a, compared with the initial electrode, the impedance of the cell increases dramatically after the first discharge. This is attributed to the discharge products generated in the cathode being poorly conductive. Unfortunately, there is not much distinct decrease in the impedance values after the first charge, which demonstrates that insulated discharge products are not completely decomposed. Combine with the SEM of first discharge and charge (Fig. 8a and b) we can clearly see the Li2O2 covered on the surface of CP where there are not have MnO2. It is normal to see the Li2O2 on the surface of CP, as the CP have the ability of oxygen catalysis ability. But the Li2O2 did not decomposed after charge which is consistent with the EIS. Fig. 7b shows the impedance of the cell is high after the 50th discharge, but have distinct decrease in the impedance values after the 50th charge. The SEM of the 50th discharge/charge (Fig. 8c and d) are consistent with the EIS (Fig. 7b).
 | ||
| Fig. 8 (a and b) SEM of the first discharge/charge, respectively. (c and d) SEM of the 50th discharge/charge, respectively. | ||
It should be noted that the electrochemical performance of pristine CP is extremely poor in a Li–O2 cell. The specific capacity normalized with the total mass of the MnO2/CP electrode is shown in Fig. 9. The Li–O2 battery with the MnO2/CP cathode and pure CP electrode without MnO2 were cycled under 0.1 mA cm−2. Compare with the pure CP without MnO2, the MnO2/CP improve the special capacity greatly.
 | ||
| Fig. 9 Discharge/charge cycling of Li–O2 cell with the MnO2/CP cathode and pure CP electrode without MnO2, based on total electrode mass. The current density is 0.1 mA cm−2. | ||
In summary, flower-like 3D nanostructured MnO2 with high porosity was grown on carbon paper using a facile electro-deposition route and was electrochemically investigated as the cathode material for rechargeable LOBs. Without the need for conductive agents and binders, the hybrid MnO2/CP delivers a high discharge capacity of 5136 mA h g−1 at a current density of 500 mA g−1 and sustained stable capacity up to 125 cycles with controlling capacity of 500 mA h g−1. The considerable catalytic performance of the hybrid was attributed to the 3D porous nanostructure, abundant oxygen defects MnO2, and facile electronic conduction in the CFP substrate that firmly supports the deposited oxide. These results indicate the promising application of MnO2/CP as self-supporting, carbon-free cathode electrocatalyst for advanced LOBs.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (U1401246, 51474110, 51474077 and 51364004) and Guangxi Nature Science Foundation (2013GXNSFDA019027 and 2013GXNSFAA019032).Notes and references
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J. M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- J. J. Wang, Y. L. Li and X. L. Sun, Nano Energy, 2013, 2, 443–467 CrossRef CAS PubMed.
- D. G. Kwabi, N. Oritz-Vitoriano, S. A. Freunberger, Y. Chen, N. Imanishi, P. G. Bruce and Y. Shao-Horn, MRS Bull., 2014, 39, 443–452 CrossRef CAS.
- Z. Y. Wen, C. Shen and Y. Lu, ChemPlusChem, 2015, 80, 270–287 Search PubMed.
- R. Black, B. Adams and L. F. Nazar, Adv. Energy Mater., 2012, 2, 801–815 CrossRef CAS PubMed.
- F. J. Li, T. Zhang and H. S. Zhou, Energy Environ. Sci., 2013, 6, 1125–1141 CAS.
- J. Lu, L. Li, J. B. Park, Y. K. Sun, F. Wu and K. Amine, Chem. Rev., 2014, 114, 5611–5640 CrossRef CAS PubMed.
- B. D. McCloskey, R. Scheffler, A. Speidel, D. S. Bethune, R. M. Shelby and A. C. Luntz, J. Am. Chem. Soc., 2011, 133, 18038–18041 CrossRef CAS PubMed.
- W. G. Fan, X. X. Guo, D. D. Xiao and L. Gu, J. Phys. Chem. C, 2014, 118, 7344–7350 CAS.
- S. T. Kim, N. S. Choi, S. J. Park and J. Cho, Adv. Energy Mater., 2015, 5, 1401030 Search PubMed.
- J. Lu, L. Cheng, K. C. Lau, E. Tyo, X. Y. Luo, J. G. Wen, D. Miller, R. S. Assary, H. H. Wang, P. Redfern, H. M. Wu, J. B. Park, Y. K. Sun, S. Vajda, K. Amine and L. A. Curtiss, Nat. Commun., 2014, 5, 4895 CrossRef CAS PubMed.
- R. Choi, J. Jung, G. Kim, K. Song, Y. Kim, S. C. Jung, Y. k. Han, H. Song and Y. M. Kang, Energy Environ. Sci., 2014, 7, 1362–1368 CAS.
- X. Xie, C. Zhang, M. B. Wu, Y. Tao, W. Lv and Q. H. Yang, Chem. Commun., 2013, 49, 11092–11094 RSC.
- J. L. Shui, F. Du, C. M. Xue, Q. Li and L. M. Dai, ACS Nano, 2014, 8, 3015–3022 CrossRef CAS PubMed.
- W. Zhou, H. Z. Zhang, H. J. Nie, Y. W. Ma, Y. N. Zhang and H. M. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 3389–3397 CAS.
- K. Zhang, X. P. Han, Z. Hu, X. L. Zhang, Z. L. Tao and J. Chen, Chem. Soc. Rev., 2015, 44, 699–728 RSC.
- D. W. Su, S. X. Duo and G. X. Wang, Sci. Rep., 2014, 4, 5767 CrossRef PubMed.
- W. Y. Zhang, Y. Zeng, C. Xu, H. T. Tan, W. L. Liu, J. X. Zhu, H. H. Hng, J. Ma, H. E. Hoster, R. Yazami and Q. Yan, RSC Adv., 2012, 2, 8508–8514 RSC.
- K. P. C. Yao, Y. C. Lu, C. V. Amanchukwu, D. G. Kwabi, M. Risch, J. G. Zhou, A. Grimaud, P. T. Hammond, F. Barde and Y. S. Horn, Phys. Chem. Chem. Phys., 2014, 16, 2297–2304 RSC.
- J. L. Shui, N. K. Karan, M. Balasubramanian, S. Y. Li and D. J. Liu, J. Am. Chem. Soc., 2012, 134, 16654–16661 CrossRef CAS PubMed.
- Y. Cao, Z. K. Wei, J. He, J. Zang, M. S. Zheng and Q. F. Dong, Energy Environ. Sci., 2012, 5, 9765–9768 CAS.
- X. F. Hu, F. Y. Cheng, X. P. Han, T. Zhang and J. Chen, Small, 2015, 7, 809–813 CrossRef PubMed.
- X. P. Han, F. Y. Cheng, C. C. Chen, Y. X. Hu and J. Chen, Nano Res., 2015, 8, 156–164 CrossRef CAS.
- J. Liu, R. Younesi, T. Gustafsson, K. Edstrom and J. F. Zhu, Nano Energy, 2014, 10, 19–27 CrossRef CAS.
- Z. L. Jian, P. Liu, F. J. Li, P. He, X. W. Guo, M. W. Chen and H. S. Zhou, Angew. Chem., Int. Ed., 2014, 53, 442–446 CrossRef CAS PubMed.
- L. L. Zhang, F. F. Zhang, G. Huang, J. W. Wang, X. C. Du, Y. L. Qin and L. M. Wang, J. Power Sources, 2014, 261, 311–316 CrossRef CAS PubMed.
- A. Riaz, K. N. Jung, W. Y. Chang, K. H. Shin and J. W. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 17815–17822 CAS.
- X. F. Hu, X. P. Han, Y. X. Hu, F. Y. Cheng and J. Chen, Nanoscale, 2014, 6, 3522–3525 RSC.
- H. Geaney, J. O'Connell, J. D. Holmes and C. O'Dwyer, J. Electrochem. Soc., 2014, 161, A1964–A1968 CrossRef CAS PubMed.
- T. N. Lambert, J. A. Vigil, S. E. White, D. J. Davis, S. J. Limmer, P. D. Burton, E. N. Coker, T. E. Beecheme and M. T. Brumbachf, Chem. Commun., 2015, 51, 9511–9514 RSC.
- L. Sun, J. F. Bu, W. X. Guo, Y. Y. Wang, M. Y. Wang and C. J. Lin, Electrochem. Solid-State Lett., 2011, 15, E1–E3 CrossRef PubMed.
- Q. Cheng, J. Tang, J. Ma, H. Zhang, N. Shinya and L. C. Qin, Carbon, 2011, 2917–2925 CrossRef CAS PubMed.
- Z. J. Su, C. Yang, C. J. Xu, H. Y. Wu, Z. X. Zhang, T. Liu, C. Zhang, Q. H. Yang, B. Lia and F. Y. Kang, J. Mater. Chem. A, 2013, 1, 12432–12440 CAS.
- R. Black, S. H. Oh, J. H. Lee, T. Yim, B. Adams and L. F. Nazar, J. Am. Chem. Soc., 2012, 134, 2902–2905 CrossRef CAS PubMed.
- S. Y. Liu, Y. G. Zhu, J. Xie, Y. Huo, H. Y. Yang, T. J. Zhu, G. S. Cao, X. B. Zhao and S. C. Zhang, Adv. Energy Mater., 2014, 4, 1301960 Search PubMed.
- H. Jang, A. Zahoor, J. S. Jeon, P. Kim, Y. S. Lee and K. S. Nahm, J. Electrochem. Soc., 2015, 162, A300–A307 CrossRef CAS PubMed.
- A. S. Poyraz, W. Q. Song, D. Kriz, C. H. Kuo, M. S. Seraji and S. L. Suib, ACS Appl. Mater. Interfaces, 2014, 6, 10986–10991 CAS.
- F. W. T. Goh, Z. L. Liu, X. M. Ge, Y. Zong, G. J. Du and T. S. A. Hor, Electrochim. Acta, 2013, 114, 598–604 CrossRef CAS PubMed.
- Y. Umezawa and C. N. Reilley, Anal. Chem., 1978, 50, 1290 CrossRef CAS.
- J. C. Carver, G. K. Schweitzer and T. A. Carlson, J. Chem. Phys., 1972, 57, 973 CrossRef CAS PubMed.
- Z. J. Su, C. Yang, C. J. Xu, H. Y. Wu, Z. X. Zhang, T. Liu, C. Zhang, Q. H. Yang, B. H. Lia and F. Y. Kang, J. Mater. Chem. A, 2013, 1, 12432–12440 CAS.
- S. Chen, G. X. Liu, H. Yadegari, H. H. Wang and S. Z. Qiao, J. Mater. Chem. A, 2015, 3, 2559–2563 CAS.
- K. Zhang, X. P. Han, Z. Hu, X. L. Zhang, Z. L. Tao and J. Chen, Chem. Soc. Rev., 2015, 44, 699–728 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15464b |
| This journal is © The Royal Society of Chemistry 2015 |