Reductant-assisted synthesis, characterization and photovoltaic characteristics of ligand-protected gold nanoparticles†
Naveed Shahzad and
Fuyi Chen*
State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xian 710072, China. E-mail: fuyichen@nwpu.edu.cn; Fax: +86 29 88492052; Tel: +86 29 88492052
First published on 16th September 2015
Abstract
Metal clusters (CLs) and nanoparticles (NPs) are promising on account of their unique properties which cannot be achieved from their bulk counterparts. Discrete electronic excitations in metal CLs and the characteristic surface plasmon resonance (SPR) phenomenon in metal NPs make them optically attractive and versatile photosensitizers in photovoltaic (PV) applications. A visible-light driven photo-electrochemical (PCE) response of a plasmonic sensitizer in a metal NP/semiconductor composite assembly can be tuned with plasmonic metal NP sizes. Therefore, it is highly desirable to explore the PEC response of a plasmonic sensitizer as a function of its NP size. In this work, a study focusing on the PEC performance of a plasmonic sensitizer as a function of its NP size has been realized through synthesizing a series of ligand-protected Au NPs at ambient conditions using various reductants, since each reductant in coordination with the ligand forms metal ion complexes which influence the reduction potentials of metals through variation in the pH of the system, and ultimately, these changes affect the reaction dynamics and tune the NP sizes and morphology. A superior PEC response of glucose-assisted synthesis of Au NPs in NP sensitized solar cells (NPSCs) with power conversion efficiency greater than 1.5% has been observed, and attributed to the relatively fine NP sizes and uniform distribution on TiO2 photoanodes. It is expected that our study will assist in exploration of different sized Au NPs in diverse applications.
1. Introduction
Dye sensitized solar cells (DSSCs) have been intensively studied worldwide, since their inception in 1991 when a group of renowned researchers laid the foundations of an efficient and low cost DSSC.1 In DSSCs, the sensitizer is one of the main components; which owing to its enhanced light absorption capability provides photo-excited electrons through interaction with light. Organic dyes, metal complexes, and perovskite compounds2,3 have widely been employed as sensitizers in DSSCs. Some dyes are expensive (ruthenium based complexes), while the other ones may pose environmental concerns (lead based complexes). These issues call for an alternative sensitizer to be explored. In recent years, metal CLs capped by stabilizing ligand are becoming fascinating materials because of their unique properties considered to be intermediate between molecular species and bulk NPs.4 Stabilizing shells or ligands such as thiolates4,5 provide stability and protection to metal CLs against oxidization, and provide a delicate control on particle sizes. PEC analysis of plasmonic metal CLs,6 their optical absorption spectra,7 and their photovoltaic properties have extensively been explored.8 Charge transfer mechanism and power conversion efficiency (PCE) of ligand-protected metal CLs greater than 2% have recently been reported in the literature.5 Apart from monometallic metal CLs, it has been demonstrated, in our previous report, that bimetallic CLs also are a unique class of photosensitizers.9 In addition to metal CLs, plasmonic metal NPs (especially Au NPs) are also regarded as promising and attractive optical materials owing to the unique phenomenon such as surface plasmon resonance10,11 and surface enhanced Raman scattering.12NPs have a wide-range of optical, electronic, catalytic, optoelectronic applications etc., where one of the important factors that can potentially influence their properties is their size and morphology.10,13 More specifically, SPR characteristics,14 extinction coefficient,15 and photocatalytic properties16 are size-depended for Au NPs, and therefore, call for the exploration of PCE response as a function of Au NPs sizes, since the visible-light driven PEC performance of plasmonic sensitizers as a function of their NPs sizes in metal NPs/semiconductor composite assembly still remains a challenge. Moreover, in Au NPs/TiO2 composite assembly, the size of Au NPs also influences the shift in equilibrium Fermi level, and thus modifies the photoinduced charge transfer efficiency in Au NPs/TiO2 composite assembly.17 Therefore, it is highly desirable to explore the PEC performance of Au NPs sensitized solar cells, as a function of NPs sizes. For stabilization of Au NPs, we have used L-glutathione ligand, since Au NPs not protected with stabilizing ligand may cause photo-corrosion, which limits the PEC performance.11 For the synthesis of different sized glutathione Au NPs (Au-GSH NPs), a widely-used metal salt reduction method has been employed on account of its simplicity, versatility and low cost. In this method, the change in synthetic conditions, such as temperature, initial concentration of reductant, and metal salt might be one effective approach to tune the sizes of Au-GSH NPs, since the complex coordination mechanisms involved in metal precursors, ligands and reducing agents provide a structural control on the nanoparticle morphology.18,19 However, in this work, the synthesis of different-sized Au-GSH NPs has been realized through the use of varied reductants at ambient conditions, leading to manifestation of their imperative role as plasmonic sensitizer in NPSCs. Nucleation and growth of Au NPs are effected by the concentration of metal ions controlled by reductants, since each reductant in coordination with ligand forms metal ion complexes which influence the reduction potentials of metals through variation in the pH of the system, and ultimately, these changes affect the reaction dynamics and tune the NPs sizes and morphology.18,19 Widely-available reductants such as sodium borohydride,6 citrates,18 glucose20 etc. are employed for the synthesis of metal NPs. A series of Au-GSH NPs has been synthesized at ambient conditions using reductants such as sodium borohydride, glucose and tri-sodium citrate to reduce AuCl3·HCl·4H2O (gold metal precursor), and demonstrated their influence on formed Au NPs sizes followed by manifestation of functional performance of the resultant different sized Au-GSH NPs as plasmonic sensitizer in NPSCs in conjunction with mesoporous TiO2 semiconductor. For convenience, Au-GSH NPs synthesized using reductants glucose, sodium borohydride, and tri-sodium citrate have been arbitrary marked as Au-GSH-B, Au-GSH-C and Au-GSH-D NPs, respectively. We also have synthesized, for comparison, Au-GSH NPs without using external reductant, and the synthesized NPs are identified as Au-GSH-A NPs. During investigation, it has been found that Au-GSH-B NPs synthesized using glucose, exhibit superior PEC response. Furthermore, optical absorption spectroscopy, electron microscopy, electrochemical impedance spectroscopy (EIS), open circuit voltage (Voc) decay method and Mott–Schottky (M–S) analysis have been conducted to elucidate the processes occurring inside NPSCs. We expect that our work on synthesis of different sized Au-GSH NPs by reductant-assisted synthesis, will contribute significantly to synthesis chemistry rendering these different sized Au-GSH NPs amenable to further exploration in a diverse range of applications, with particular examples being their exploration coupled with suitable dyes in photovoltaic cells,21 synergistic effects of Au NPs and CLs to enhance the optical activity in energy applications,22,23 size-dependent extinction coefficient of Au NPs,15 and tuning the photodynamic therapy and activity through different sized Au NPs in medical applications.24
2. Experimental section
All reagents were used as analytical grades without any further purification. We synthesize mesoporous TiO2 (mTiO2), glutathione-protected Au NPs, Pt counter electrode, and fabricate NPs sensitized solar cells.2.1 Synthesis of TiO2 colloidal solution and ligand protected gold NPs
Mesoporous TiO2 has been synthesized according to the method published in the literature.25 We employ sol-hydrothermal synthesis for TiO2 colloidal solution. The details of the synthesis of mTiO2 have been mentioned in the ESI.† Au-GSH-A NPs have been synthesized according to the literature with a little modification26 and Au-GSH-B, Au-GSH-C and Au-GSH-D NPs by following the procedure mentioned in the literature with some modification in the use of reductants.6 ESI† can be viewed for gold NPs synthesis.2.2 Fabrication of NPSCs
To explore the photo-electrochemical performance of these ligand-protected gold NPs synthesized at ambient conditions using different reductants, we follow the procedure reported in the literature5 to fabricate NPSCs. Mesoporous TiO2 spin-coated on FTO and modified with Au-GSH NPs has been used as photoanode and Pt coated FTO as counter electrode in the presence of cobalt-based redox shuttle. Schematically the structure of NPs sensitized solar cells have been shown in the Fig. 1.Fabrication of TiO2 photoanodes, Pt counter electrode and assembly of NPSCs has been described, in details, in the ESI.†
2.3 Characterization
The TiO2 photoanodes were characterized by SEM (JSM, 6390A) with energy dispersive X-ray spectroscopy (SEM-EDS) for surface morphology. X-ray diffraction (XRD) technique was employed to determine the crystallite size of TiO2 and its crystal phase. The XRD data were obtained in the 2θ range between 20 and 70° using an X'Pert-PRO diffractometer. HRTEM and selected area electron diffraction (SAED) (TECNAI instrument) were conducted to characterize the NPs. Optical absorption spectra were obtained using Zolix Omni-λ 300 Monochromator/Spectrograph and DCS103 Data Acquisition System. All PEC measurements were conducted under visible light generated through 300 W Xenon lamp with 90 mW cm−2 incident power using CHI660C electrochemical workstation. EIS, Voc decay method and M–S analysis were performed on assembled devices using Co(II)/Co(III) redox couple on CHI660C electrochemical workstation. Active area of each device was marked as 0.145 cm2. EIS was performed under dark at Voc using 10 mV amplitude and frequency in the range of 1 Hz to 100 kHz. The resultant spectra were fitted by using ZView software. M–S plots were obtained at amplitude of 10 mV with voltage in the range of +1 to −1 V.3. Results and discussion
3.1 Optical absorption spectroscopy of Au-GSH NPs
Plasmonic metals such as Au, Ag, Cu etc. usually possess a sharp absorption peaks which are due to their localized surface plasmon resonance (LSPR) phenomenon arising from the collective oscillations of free electrons in response to an incident light.27 Optical absorption spectra of dispersions of Au-GSH-A, Au-GSH-B, Au-GSH-C and Au-GSH-D NPs have been presented in Fig. 2. Intensity of absorption signals are shown in Fig. 2a, where the maximum intensity of absorption signals of Au-GSH-B and Au-GSH-C NPs is observed to be red-shifted in comparison with that of Au-GSH-A NPs and Au-GSH-D NPs indicating a wide range of optical absorption. These absorption signals are based on only visible range of solar spectrum. Different NPs exhibit different absorption signal intensity indicating a varied response to visible light. Maximum intensity of absorption signals is found to be at 410 nm, 465 nm, 470 nm and 455 nm for Au-GSH-A, Au-GSH-B, Au-GSH-C and Au-GSH-D NPs, respectively. Before the wavelength of 400 nm; all four types of Au-GSH NPs exhibit almost similar absorption trend, however, beyond 400 nm the absorption is distinctly different from one another suggesting a wide absorption band extending towards visible region having longer wavelengths. Fig. 2b shows the optical absorption spectra where Au-GSH-C NPs exhibit a wide absorption band between the wavelengths of 450 nm to 700 nm owing to the LSPR effect of Au NPs. The SPR peak of Au-GSH-C NPs is not very sharp since the NPs sizes are very small. The SPR peak of Au NPs shifts towards longer wavelengths with an increase in particle size. In Au-GSH-C NPs, the LSPR peak is centered at 550 nm, slightly red-shifted than that observed in Au NPs and gold nanowires reported in the literature.28 Such wide SPR absorption band between the wavelengths of 450 nm to 700 nm has not been observed in Au-GSH-A, Au-GSH-B and Au-GSH-D NPs. Moreover, optical absorbance is influenced by the NPs size and particle volume. Different sizes of metal NPs exhibit varied absorption bands in optical absorption spectra. As the NPs size increases, their band gapes become smaller and corresponding absorption spectra extends towards longer wavelength.29 | ||
| Fig. 2 Optical absorption spectra of Au-GSH NPs dispersed in DI water, (a) intensity of optical absorption signals, and (b) optical absorbance spectra. | ||
3.2 Au-GSH NPs sizes and morphology
Transmission electron microscopy has been conducted on Au-GSH-A, Au-GSH-B, Au-GSH-C and Au-GSH-D NPs for the characterization of NPs morphology. TEM images are shown in the Fig. 3. TEM image of Au-GSH-A NPs (Fig. 3a) synthesized without any reductant has not been resolved appreciably which might be due to incomplete formation of Au NPs, since we synthesize all types of NPs at room temperature. Au-GSH-B NPs (Fig. 3b) employing glucose reductant have core size in the range of 3–4 nm, whereas, Au-GSH-C NPs (Fig. 3c) exhibit the core size in the range of 4–6 nm. Au-GSH-D NPs (Fig. 3d) exhibit relatively larger NPs size likely due to agglomeration of individual NPs synthesized using tri-sodium citrate. Histograms showing the approximate distribution of NPs sizes have been shown in the Fig. S1.† High-resolution TEM has been performed for typically Au-GSH-C NPs to determine interfringe distances. In HRTEM image (Fig. 3e), the interfringe distances of Au-GSH-C NPs have been measured to be around 0.24 nm, indicating the (111) plane spacing for FCC gold. These interfringe distances are similar to Au-GSH-B and Au-GSH-D NPs, however, the HR-TEM could not resolve interfringe distances for Au-GSH-A NPs. Selected area electron diffraction (SAED) patterns have been obtained for Au-GSH-B and Au-GSH-C NPs, and are displayed as inset in the corresponding TEM images, which suggest the polycrystalline nature of Au-GSH NPs. Au-GSH-A NPs show lack of clear lines of demarcation between the individual NPs, which provide hurdles in the analysis of HR-TEM. Furthermore, EDX analysis of relatively finer NPs i.e. Au-GSH-B NPs has been performed and presented in Fig. 3f which indicates the presence of gold. The presence of Ni in EDX pattern shows the peaks of Ni substrate used for TEM analysis.3.3 Morphology of mTiO2 modified with Au-GSH NPs
TiO2 semiconductor has been selected as a working electrode in NPSCs. Mesoporous morphology of TiO2 has been obtained through controlled sintering process. XRD corroborates the presence of anatase phase of TiO2 synthesized through sol-hydrothermal method. Anatase phase of TiO2 and its mesoporous morphology are usually preferred in comparison with other phases and dense morphology.30 XRD pattern of spin-coated mTiO2 and its film thickness on FTO have been shown in Fig. 4a and b, respectively. Since XRD has been performed on TiO2 coated FTO glass, therefore, FTO peaks are also present in Fig. 3a. The anatase TiO2 peaks are marked by the symbol (♦) in accordance with JCPDS # 21-1272, whereas the peaks marked by the symbol (Δ) are assigned to FTO substrate according to JCPDS # 46-1088. The crystallite size of mTiO2 can be estimated from a famous Scherrer's equation: L = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where L is the crystallite size, λ is the wavelength of the X-ray radiation (CuKα radiations have been used with the wavelength of 0.1542 nm), K is usually taken as 0.89, and β is the line width at half-maximum height (FWHM).31 From Fig. 4a, the crystallite size has been determined as 16.7 nm for spin-coated mTiO2. TiO2 photoanodes were coated with Au-GSH NPs through immersion or soaking technique. XRD was performed on NPs-coated TiO2 photoanodes to determine the peaks of Au NPs (not shown here). Au peaks have not been observed in XRD patterns of Au NPs-coated TiO2, which indicate a small amount of NPs that have been used to modify mTiO2 and also the uniform distribution of metal NPs in mTiO2. Thickness of spin-coated mTiO2 film on FTO has been shown in Fig. 4b, where a cross-sectional image has been taken by SEM. The thickness has been observed to be in the range of 4–5 microns. The synthesized Au-GSH NPs have been deposited on mTiO2 photoanodes through immersion technique. The concentration of Au-GSH NPs in DI water was adjusted between 0.60–0.70 by wt% in each case, and same immersion time for each photoanode in Au-GSH NPs dispersions, indicates a uniform loading of metal NPs on the photoanodes.22 The pH value should be between 2–6. In this pH range, the –COO− group of glutathione molecule is electrostatically bonded with the positively charged TiO2.32 The pH value has been controlled within the above mentioned range to ensure fair comparison of each plasmonic sensitizer. The adjustment in pH was made through the addition of NaOH and acetic acid.
θ, where L is the crystallite size, λ is the wavelength of the X-ray radiation (CuKα radiations have been used with the wavelength of 0.1542 nm), K is usually taken as 0.89, and β is the line width at half-maximum height (FWHM).31 From Fig. 4a, the crystallite size has been determined as 16.7 nm for spin-coated mTiO2. TiO2 photoanodes were coated with Au-GSH NPs through immersion or soaking technique. XRD was performed on NPs-coated TiO2 photoanodes to determine the peaks of Au NPs (not shown here). Au peaks have not been observed in XRD patterns of Au NPs-coated TiO2, which indicate a small amount of NPs that have been used to modify mTiO2 and also the uniform distribution of metal NPs in mTiO2. Thickness of spin-coated mTiO2 film on FTO has been shown in Fig. 4b, where a cross-sectional image has been taken by SEM. The thickness has been observed to be in the range of 4–5 microns. The synthesized Au-GSH NPs have been deposited on mTiO2 photoanodes through immersion technique. The concentration of Au-GSH NPs in DI water was adjusted between 0.60–0.70 by wt% in each case, and same immersion time for each photoanode in Au-GSH NPs dispersions, indicates a uniform loading of metal NPs on the photoanodes.22 The pH value should be between 2–6. In this pH range, the –COO− group of glutathione molecule is electrostatically bonded with the positively charged TiO2.32 The pH value has been controlled within the above mentioned range to ensure fair comparison of each plasmonic sensitizer. The adjustment in pH was made through the addition of NaOH and acetic acid.
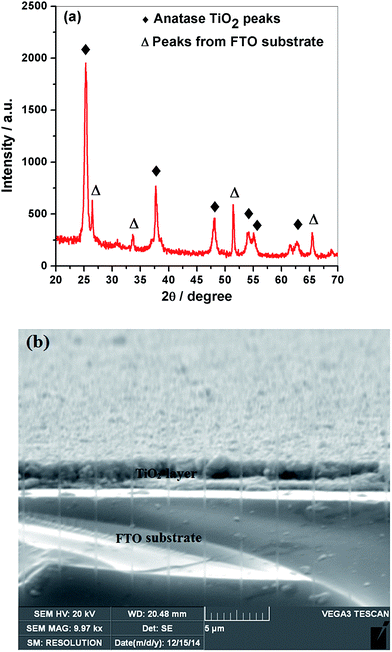 | ||
| Fig. 4 (a) XRD pattern of spin-coated TiO2 photoanode, and (b) SEM cross-sectional view of spin-coated mTiO2 film thickness on FTO substrate. | ||
SEM images of mTiO2 photoanodes modified with Au-GSH-A, Au-GSH-B, Au-GSH-C and Au-GSH-D NPs are shown in Fig. 5a–d, respectively. The EDX spectra of mTiO2 modified with Au-GSH NPs are presented in the Fig. S2(e–h)† along with corresponding SEM images (Fig. S2(a–d)†). EDX spectra clearly indicate the presence of Au NPs on mTiO2 semi-conductor. SEM morphology is apparently different in each mTiO2 photoanode modified by different Au-GSH NPs, suggesting a variation in metal NPs distribution on TiO2 photoanodes. It is expected that the variation in metal NPs distribution on TiO2 photoanodes is due to deposition of different sized Au-GSH NPs with varied morphology, which may change the PEC performance of these plasmonic sensitizers.
 | ||
| Fig. 5 SEM images of (a) mTiO2 modified with Au-GSH-A NPs, (b) mTiO2 modified with Au-GSH-B NPs, (c) mTiO2 modified with Au-GSH-C NPs, and (d) mTiO2 modified with Au-GSH-D NPs. | ||
3.4 Photo-electrochemical response of Au-GSH NPs as photo-sensitizers
PEC response depends on loading amount of sanitizers or dyes on metal oxide semiconductor coated on transparent conductive oxide (TCO). To ensure the same loading amount of Au NPs on TiO2 photoanodes in each case, we have maintained the concentration of Au NPs in water between 0.60–0.70 wt%. Equal immersion time was given to each TiO2 photoanode so as to ensure same loading amount of sensitizer for a fair comparison, since the change in immersion time modifies the loading amount of sensitizer on photoanodes.22 Active area of solar cells is same, therefore, it is expected that, given the same loading of sensitizers, the change in PEC response of NPSCs might be due to different sized Au-GSH NPs. PEC response of NPSCs fabricated from Au-GSH-A, Au-GSH-B, Au-GSH-C and Au-GSH-D NPs has been demonstrated in Fig. 6. Fig. 6a demonstrates J–V characteristics of Au-GSH NPs sensitizers under both dark and illumination conditions. Au-GSH-B NPs show the superior performance delivering short circuit current density (Jsc) of 4.15 mA cm−2, Voc of 692 mV, FF of 45% and PCE of 1.53%. Au-GSH-C NPs deliver Jsc of 4.08 mA cm−2, Voc of 615 mV, FF of 42% and PCE of 1.26%. The higher values of Jsc in NPSCs constructed from Au-GSH-B and Au-GSH-C NPs are attributed to their higher light absorption. Relatively finer NPs size in Au-GSH-B NPs is likely one of the main factors that favor high Jsc on account of effective charge-carrier separation. Furthermore, smaller Au NPs shift the Fermi level more to the negative side than that of larger Au NPs, and improves the charge transfer efficiency.16 It is expected that larger NPs (Au-GSH-D) might reduce the charge-carrier separation, and thus the PEC performance. Au-GSH-C NPs as sensitizers have a little higher dark current density in comparison with Au-GSH-B NPs which slightly degrades its performance and restricts the PCE up to 1.26%. Dark current densities have also been shown in the Fig. 6a. One can view that the dark current density is slightly larger in Au-GSH-C NPLs sensitizers (Fig. 6a).Lower PEC performance of Au-GSH-A NPs sensitizers is expected due to their lower light absorption capability and incomplete formation of metal CLs and NPs at 25 °C because their synthesis temperature is lower than what is required (70 °C) for the formation of GSH-protected Au CLs.5,26 If Au-GSH-A NPs are synthesized at 70 °C, the metal salt precursors in the presence of ligands are reduced, and results in the formation of CLs, since some ligands can perform dual role: capping agent and reducing agent.19 Au-GSH-D NPs have some agglomeration effect which might be responsible for lower PEC performance. Fig. 6b manifests the behavior of Jsc with time for all NPSCs under investigation. When the illumination is off, the photocurrent in NPSCs approaches zero which again starts generating in the device when the illumination is on. The performance of NPSCs under illumination on/off cycle indicates the decay of Jsc. The decay of Jsc is less pronounced in Au-GSH-B NPs sensitizers. The results of photo-electrochemical measurements are summarized in Table 1.
| NPSCs | Jsc (mA cm−2) | Voc (mV) | FF (%) | Efficiency (%) |
|---|---|---|---|---|
| Au-GSH-A | 1.96 | 440 | 40 | 0.50 |
| Au-GSH-B | 4.15 | 692 | 45 | 1.53 |
| Au-GSH-C | 4.08 | 615 | 42 | 1.26 |
| Au-GSH-D | 3.52 | 636 | 44 | 1.22 |
The superior photovoltaic characteristics of glucose-assisted synthesis of Au-GSH-B NPs are assigned to relatively finer NPs morphology. The results presented in the Table 1 for NPSCs are relatively lower than those reported in the literature.5 The potential reason for the lower performance, in our study, might be attributed to intrinsically different characteristics of Au NPs (in our study) and extremely small Au CLs reported in the literature.5 Another possible reason is likely the lower loading amount of plasmonic sensitizers. Since, the main focus of the present study is to explore the imperative role of different sized Au NPs as plasmonic sensitizer on the PEC performance, which has been realized through employing varied reductant and synthesizing a series of Au NPs with different sizes at ambient conditions.
3.5 Electron recombination kinetics and lifetimes
To investigate the processes occurring inside NPSCs, we perform electrochemical impedance spectroscopy. EIS is a steady-state method which has become a powerful tool to investigate the electrochemical and photo-electrochemical processes taking place in dye-sensitized solar cells. EIS generates Nyquist plots which normally represent two or three semicircles in the order of increasing frequency. These semi-circles are usually assigned to the Nernst diffusion within the electrolyte, the electron transfer at the semiconductor/electrolyte interface, and the redox reaction taking place at the counter electrode.33 Electrochemical impedance spectroscopy has been conducted under dark and graphically represented in Fig. 7. Fig. 7a and b show the Nyquist plots, whereas, Fig. 7c shows the Bode phase plots under dark conditions. Fig. 7b shows the Nyquist plots at an extended scale. The corresponding equivalent circuit has been shown in the Fig. S3† which represents the processes taking place in a typical liquid DSSC.From the equivalent circuit, Rpt, in the high frequency region, indicates the resistance given by the sum of the CE/electrolyte and the FTO/TiO2 interface. Rrc at medium frequency range is associated with the recombination interfacial resistance at TiO2/NPs/electrolyte interface. A small lowest frequency arc is generally attributed to the impedance of diffusion of redox species in the electrolyte i.e. Warburg diffusion (Ws). In our EIS experiment, Rrc is dominated Ws and Rpt because charge-transfer is the main process occurring at TiO2/NPs/electrolyte interface. Finally, Rs is the displacement of the arc which is attributed to the total series resistance of the solar cell.34 We, herein, focus mainly on the middle frequency arc, since an identical Pt CE and same electrolyte have been used in the investigation. Therefore, it is expected that the change occurs only at TiO2/Au-GSH-NPs/electrolyte interface. The smallest middle frequency arc diameter for NPSC constructed from Au-GSH-A NPs sensitizers indicates the lowest resistance at the TiO2/Au-GSH-NPs/electrolyte interface, suggesting a higher recombination rate. NPSCs fabricated from TiO2 modified with Au-GSH-B NPs have higher value of FF which may be due to its lowest series resistance because Rs has direct influence on fill factor.35 During synthesis of gold NPs, we used L-glutathione as a capping agent. We suppose that L-glutathione has same role in electron transfer kinetics and recombination dynamics, since ligand layer is expected to be present in each case.
Electron capturing by oxidizing species in the electrolyte is one of the main performance limiting phenomena. Due to a simple kinetic competition between charge collection and recombination; the power conversion efficiency of NPSC reduces when electron recombination rate accelerates and results in shorter electron lifetime. Moreover, we can make a comparison of the electron recombination lifetime or relaxation time constant from the Bode plots according to the relation: τr = 1/ωmax = 1/2πfmax, where, fmax is the maximum frequency of the mid-frequency peak which has an inverse relation with electron lifetime i.e. τr.36 The electron recombination lifetime is smaller for Au-GSH-A NPs consistent with the smallest arc diameter in the Nyquist plot. Furthermore, the peak frequency from the Bode phase diagrams is usually attributed to the recombination resistance.33 The characteristic peak frequency shifts towards lower frequency which shows the longer electron lifetimes for Au-GSH-B NPs sensitizers (Fig. 7c). EIS measurements show that Au-GSH-B NPs sensitizers demonstrate lower series resistance, lower recombination rate and longer electron lifetime, which collectively favor the superior performance of Au-GSH-B NPs. Parameters evaluated from EIS spectra for each NPs type has been mentioned in Table 2. The calculated electron lifetimes are slightly larger than those reported in the literature for TiO2 modified with Ag, largely due to different cell configuration and measuring conditions.37
| NPSCs | Rs (Ω) | Characteristic frequency (Hz) | Electron lifetime (ms) | Flat band potential (V) | ND (conc. cm−3) |
|---|---|---|---|---|---|
| Au-GSH-A | 28.35 | 1170 | 0.136 | −0.76 | 2.39883 × 1021 |
| Au-GSH-B | 26.95 | 371 | 0.428 | −0.89 | 2.13229 × 1022 |
| Au-GSH-C | 28.04 | 547 | 0.290 | −0.85 | 1.06615 × 1022 |
| Au-GSH-D | 27.10 | 459 | 0.346 | −0.78 | 7.46302 × 1021 |
To further investigate the electron recombination dynamics, we perform open-circuit voltage decay method. Fig. 8 shows the transient open circuit voltage decay curves for all the NPs under investigation. In this method, at illumination, the solar cell has some Voc, which decays when illumination is switched off. Light source is suddenly turned off and allowed the test to continue at dark and monitored the decay of Voc with time. Decay of Voc is sluggish in Au-GSH-B NPs in comparison with Au-GSH-C and Au-GSH-D NPs. The sluggishness in Voc decay in Au-GSH-B NPs indicates the lower recombination rate kinetics. The steeper decay in Au-GSH-C NPs indicates the slightly higher recombination of photo-electrons.
3.6 Flat band potentials and carrier concentrations
The concentration of electrons in TiO2 conduction band (CB) and the corresponding flat band potentials (EFB) are important parameters which need to be calculated at the junctions formed by Au metal and TiO2 semiconductor. M–S analysis has been conducted to acquire the data for these parameters. Space charge capacitances are estimated from M–S plots so as to calculate donor density (ND) and EFB, according to the following eqn (1).38| Csc−2 = 2/qεεoNDA2(E − EFB − kT/q) | (1) |
Fig. 9 shows the M–S plots for all sensitizers under study. One can observe the trend in slopes of linear regions of TiO2 modified with different Au-GSH NPs. The obvious decline in the slope is an indicative of increasing carrier concentration.
 | ||
| Fig. 9 Mott–Schottky plots at 10 mV amplitude for NPSCs fabricated by employing mTiO2 modified with Au-GSH NPs plasmonic sensitizer. | ||
The value of EFB and ND are mentioned in Table 2. EFB becomes more negative for TiO2 photoanodes modified with Au-GSH-B NPs. Highest Jsc obtained in Au-GSH-B NPs sensitized solar cells is likely due to its higher donor density, resulting from its higher light absorption and effective charge-carrier separation. It is expected that the shift in the Fermi level is more negative in Au-GSH-B NPs (finer NPs), with an attendant increase in carrier concentration in TiO2 conduction band and improves the charge transfer efficiency.16 The absorption is highest in Au-GSH-C NPs, but the relatively lower carrier concentration than that of Au-GSH-B NPs indicates a bit higher electron recombination rate. The lower carrier concentration due to electron recombination in Au-GSH-C NPs is consistent with its shorter electron lifetime and lower Rrc. The lower carrier concentration in Au-GSH-A and Au-GSH-D NPs is likely due to its lower optical absorption. The superior PEC performance manifested by Au-GSH-B NPs sensitizers may be due to its lowest series resistance, highest optical absorption and highest donor density. Higher light absorption of Au-GSH-B NPs suggests its higher light harvesting efficiency (LHE), since the LHE is governed by the optical absorption, therefore, the higher Jsc in Au-GSH-B NPs sensitizers.40
The mechanism of charge movement in NPSC has been schematically demonstrated in Fig. 10.
 | ||
| Fig. 10 Schematic representation of the mechanism for the movement of charges in NPSCs with Au-GSH NPs as plasmonic sensitizer. | ||
It is well-known that Au NPs having diameters ranging from 1–10 nm would display electronic band structures owing to quantum mechanical rule.10 It has also been reported that Au NPs with sizes in the range of 5–20 nm show a characteristic SPR phenomenon with the position of absorption peak varying with NPs morphology and NPs coating.28 Virtually, Au NPs having these sizes should function as a sensitizer, since SPR behavior extends the light absorption capability through absorbing lower energy photons.
From the mechanism view points, the Fermi energy (Ef) of Au is lower than that of TiO2, therefore, the movement of electrons takes place from TiO2 to gold to equilibrate the Fermi levels. A Schottky barrier is formed owing to Fermi energy alignment, thus, bending the valence band and conduction bands upward.41 This transfer of electrons to gold causes the shift in Fermi-level in Au NPs/TiO2 composite assembly to more negative potential, equilibrating the Fermi level with that of TiO2. This shift in Fermi level towards negative potentials causes the photo-generated charge transfer efficiency in Au NPs/TiO2 composite assembly.17 As shown schematically in Fig. 10, upon visible-light irradiation, SPR-excitation generates photoelectrons which are injected into TiO2 CB overcoming the energy barrier.11 Apart from SPR-excitation, the local electromagnetic field enhancement through SPR phenomenon is another important phenomenon which reinforces the charge carrier generation by effectively separating them.42 The injected electrons are transported through TiO2 CB to the current collector and reduce redox-couple at the counter electrode (CE). CE and redox-couple in the electrolyte are at the same potentials, and voltage between the donors in the electrolyte and the TiO2 CB is a constant, therefore, the Voc of the working electrode is possibly determined by the number of photoelectrons injected from Au NPs to TiO2 CB. The higher the quantity of injected photoelectrons, the larger would be the resultant Voc in the system. The higher ND value observed in Au-GSH-B NPs sensitizers results in higher Voc consistent with the J–V characteristics. Higher ND value shifts the quasi-Fermi level upward, which results in larger open circuit voltage. The current generation in the actual working conditions of metal NPs-sensitized solar cells, the cell's generated constant current is equal to the sum of photo-generated current and dark current minus the current owing to back reactions and leakage or shunting. The PEC performance of all sensitizers under investigation is not very high, since the loading of sensitizer on TiO2 is not high enough owing to the low concentration of metal NPs (0.60–0.70 wt% in DI water), and the absence of TiO2 blocking layer on FTO. The TiO2 blocking layer is beneficial to reduce the electron recombination as reported by elsewhere.43
In summary, the Au-GSH-B NPs synthesized using glucose as a reductant are more efficient in comparison with Au-GSH-A, Au-GSH-C and Au-GSH-D NPs; predominately, owing to the higher optical absorption, lowest series resistance and highest donor density. The superior PEC response indicates that glucose in coordination with glutathione ligand assists in the formation of finer NPs and their uniform distribution with minimal aggregation, which ultimately result in higher PEC performance. Our experimental study reveals that PEC performance of plasmonic sensitizer varies with their NPs sizes, and the efforts are under way to couple these different sized Au NPs with some suitable dyes to optimize the photovoltaic characteristics in dye-sensitized solar cells.
4. Conclusions
We have successfully synthesized and characterized Au-GSH NPs having varied sizes using different reductants at ambient conditions, followed by manifestation of their functional performance as plasmonic sensitizer in NPs-sensitized solar cells. The reaction dynamics and resultant NPs morphology are influenced by the interaction of reductant with ligand. Superior PEC response was observed in glucose-assisted synthesis of Au-GSH NPs, and attributed to the relatively finer NPs sizes and uniform distribution on TiO2 photoanodes, resulting in an improved power conversion efficiency greater than 1.5%. The higher optical absorption, lowest series resistance, sluggish electron recombination kinetics and highest carrier concentration have further corroborated the imperative role of finer Au-GSH NPs towards improving the PEC performance. We expect that our study contribute significantly to synthesis chemistry and assists in exploration of different sized metal NPs in a diverse range of energy and medical applications.Conflict of interest
The authors declare no competing financial interests.Acknowledgements
This study has been supported by the National Natural Science Foundation of China (grant no. 51271148 and 50971100), the Research Fund of State Key Laboratory of Solidification Processing in China (grant no. 30-TP-2009), and the Aeronautic Science Foundation Program of China (grant no. 2012ZF53073), and the Doctoral Fund of Ministry of Education of China (grant no. 20136102110013).References
- B. Oregan and M. Gratzel, Nature, 1991, 353, 737–740 CrossRef CAS PubMed.
- M. Gratzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef CAS.
- W. Zhang, M. Saliba, S. D. Stranks, Y. Sun, X. Shi, U. Wiesner and H. J. Snaith, Nano Lett., 2013, 13, 4505–4510 CrossRef CAS PubMed.
- M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2008, 130, 5883–5885 CrossRef CAS PubMed.
- Y. S. Chen, H. Choi and P. V. Kamat, J. Am. Chem. Soc., 2013, 135, 8822–8825 CrossRef CAS PubMed.
- A. Kogo, N. Sakai and T. Tatsuma, Nanoscale, 2012, 4, 4217–4221 RSC.
- E. B. Guidez, V. Makinen, H. Hakkinen and C. M. Aikens, J. Phys. Chem. C, 2012, 116, 20617–20624 CAS.
- N. Sakai, S. Nakamura and T. Tatsuma, Dalton Trans., 2013, 42, 16162–16165 RSC.
- N. Shahzad, F. Chen, L. He, W. Li and H. Wang, J. Power Sources, 2015, 294, 609–619 CrossRef CAS PubMed.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- Y. Tian and T. Tatsuma, J. Am. Chem. Soc., 2005, 127, 7632–7637 CrossRef CAS PubMed.
- S. J. Oldenburg, S. L. Westcott, R. D. Averitt and N. J. Halas, J. Chem. Phys., 1999, 111, 4729–4735 CrossRef CAS PubMed.
- X. C. Pang, L. Zhao, W. Han, X. K. Xin and Z. Q. Lin, Nat. Nanotechnol., 2013, 8, 426–431 CrossRef CAS PubMed.
- H. J. Kim, S. H. Lee, A. A. Upadhye, I. Ro, M. I. Tejedor-Tejedor, M. A. Anderson, W. B. Kim and G. W. Huber, ACS Nano, 2014, 8, 10756–10765 CrossRef CAS PubMed.
- X. O. Liu, M. Atwater, J. H. Wang and Q. Huo, Colloids Surf., B, 2007, 58, 3–7 CrossRef CAS PubMed.
- V. Subramanian, E. E. Wolf and P. V. Kamat, J. Am. Chem. Soc., 2004, 126, 4943–4950 CrossRef CAS PubMed.
- M. Jakob, H. Levanon and P. V. Kamat, Nano Lett., 2003, 3, 353–358 CrossRef CAS.
- X. H. Ji, X. N. Song, J. Li, Y. B. Bai, W. S. Yang and X. G. Peng, J. Am. Chem. Soc., 2007, 129, 13939–13948 CrossRef CAS PubMed.
- N. Ortiz and S. E. Skrabalak, Langmuir, 2014, 30, 6649–6659 CrossRef CAS PubMed.
- M. Mohl, P. Pusztai, A. Kukovecz, Z. Konya, J. Kukkola, K. Kordas, R. Vajtai and P. M. Ajayan, Langmuir, 2010, 26, 16496–16502 CrossRef CAS PubMed.
- Z. H. Chen, Y. B. Tang, C. P. Liu, Y. H. Leung, G. D. Yuan, L. M. Chen, Y. Q. Wang, I. Bello, J. A. Zapien, W. J. Zhang, C. S. Lee and S. T. Lee, J. Phys. Chem. C, 2009, 113, 13433–13437 CAS.
- F. X. Xiao, Z. Zeng and B. Liu, J. Am. Chem. Soc., 2015, 137, 10735–10744 CAS.
- K. G. Stamplecoskie and P. V. Kamat, J. Phys. Chem. Lett., 2015, 6, 1870–1875 CrossRef CAS PubMed.
- W. Jiang, B. Y. S. Kim, J. T. Rutka and W. C. W. Chan, Nat. Nanotechnol., 2008, 3, 145–150 CrossRef CAS PubMed.
- J. H. Wu, G. T. Yue, Y. M. Xiao, J. M. Lin, M. L. Huang, Z. Lan, Q. W. Tang, Y. F. Huang, L. Q. Fan, S. Yin and T. Sato, Sci. Rep., 2013, 3, 1283 Search PubMed.
- Z. T. Luo, X. Yuan, Y. Yu, Q. B. Zhang, D. T. Leong, J. Y. Lee and J. P. Xie, J. Am. Chem. Soc., 2012, 134, 16662–16670 CrossRef CAS PubMed.
- T. Udayabhaskararao, M. S. Bootharaju and T. Pradeep, Nanoscale, 2013, 5, 9404–9411 RSC.
- H. Yu, M. Chen, P. M. Rice, S. X. Wang, R. L. White and S. H. Sun, Nano Lett., 2005, 5, 379–382 CrossRef CAS PubMed.
- H. Y. Yang, Y. Wang, H. Q. Huang, L. Gell, L. Lehtovaara, S. Malola, H. Hakkinen and N. F. Zheng, Nat. Commun., 2013, 4, 2422 Search PubMed.
- W. Shao, F. Gu, C. Z. Li and M. K. Lu, Ind. Eng. Chem. Res., 2010, 49, 9111–9116 CrossRef CAS.
- Q. H. Zhang, L. Gao and J. K. Guo, Appl. Catal., B, 2000, 26, 207–215 CrossRef CAS.
- N. Sakai and T. Tatsuma, Adv. Mater., 2010, 22, 3185–3188 CrossRef CAS PubMed.
- X. Zhang, F. Liu, Q. L. Huang, G. Zhou and Z. S. Wang, J. Phys. Chem. C, 2011, 115, 12665–12671 CAS.
- C. Longo, A. F. Nogueira, M. A. De Paoli and H. Cachet, J. Phys. Chem. B, 2002, 106, 5925–5930 CrossRef CAS.
- B. L. He, X. Meng and Q. W. Tang, ACS Appl. Mater. Interfaces, 2014, 6, 4812–4818 CAS.
- S. Lee, J. H. Noh, H. S. Han, D. K. Yim, D. H. Kim, J. K. Lee, J. Y. Kim, H. S. Jung and K. S. Hong, J. Phys. Chem. C, 2009, 113, 6878–6882 CAS.
- S. P. Lim, A. Pandikumar, N. M. Huang and H. N. Lim, RSC Adv., 2014, 4, 38111–38118 RSC.
- D. A. M.-S. Hyoung-il Kim, W. Kim and W. Choi, Energy Environ. Sci., 2015, 8, 247–257 Search PubMed.
- J. Y. Kim, H. S. Jung, J. H. No, J. R. Kim and K. S. Hong, J. Electroceram., 2006, 16, 447–451 CrossRef CAS.
- K. Miettunen, J. Halme, A. M. Visuri and P. Lund, J. Phys. Chem. C, 2011, 115, 7019–7031 CAS.
- A. Stevanovic, S. L. Ma and J. T. Yates, J. Phys. Chem. C, 2014, 118, 21275–21280 CAS.
- Z. W. Liu, W. B. Hou, P. Pavaskar, M. Aykol and S. B. Cronin, Nano Lett., 2011, 11, 1111–1116 CrossRef CAS PubMed.
- K. Miettunen, J. Halme, P. Vahermaa, T. Saukkonen, M. Toivola and P. Lund, J. Electrochem. Soc., 2009, 156, B876–B883 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15414f |
| This journal is © The Royal Society of Chemistry 2015 |





