Synthesis, optical, electrochemical properties and photovoltaic performance of novel panchromatic meso-thiophene BODIPY dyes with a variety of electron-donating groups at the 3,5-positions†
Junxu Liaoa,
Yongjun Xu*b,
Hongbin Zhaob,
Yucai Wangb,
Wentao Zhangb,
Fei Pengb,
Shengjie Xieb and
Xiaoxi Yangab
aSchool of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510641, People's Republic of China
bCollege of Energy and Chemical Engineering, Dongguan University of Technology, Dongguan 523808, People's Republic of China. E-mail: hnllxyj@126.com
First published on 7th October 2015
Abstract
A series of novel BODIPY derivatives (BDP1–5) containing a thiophene motif at the meso-position and various electron-donating units at the 3,5-positions were designed and synthesized. With the extended D–A–D conjugated skeleton, these dyes exhibit effective panchromatic absorption with maximum extinction coefficients over 1.4 × 105 M−1 cm−1 in solution state and dramatically higher values in the film state. Suitable HOMO/LUMO energy levels, around −5.4 eV and −3.8 eV, respectively, vs. PCBM combined with the good solubility and perfect film-forming ability make them attractive for BHJ solar cell applications. The photovoltaic device based on BDP2 and PC61BM exhibits a potential power conversion efficiency of 2.12%, a moderate open-circuit voltage of 0.73 V and a relatively high short-circuit current density of 7.64 mA m−2.
Introduction
Absorption of incident light is the preliminary process in the function of an organic photovoltaic (OPV) device.1,2 It can be said that the material's absorption ability significantly affects the device performance. A qualitative analysis of the solar irradiance spectrum reveals that more than 60% of the solar photon flux density lies between 300–900 nm of the visible spectrum.2 Arguably, efficient light absorbers must therefore have absorption spectra that overlap with the solar irradiance spectrum in the visible region to harness most of the incident sunlight. An attractive strategy to obtain high-efficient OPV devices is designing panchromatic materials with reduced optical band gaps to encompass more of the visible and near IR region of the sun-light spectrum.3–9 Another key factor for OPV donor materials is that they should possess commensurately aligned frontier molecular orbitals with acceptor materials to enable efficient charge separation at the donor–acceptor interface and provide large open circuit voltage.1,4,10,11 However, the challenge is the fact that tuning of the optical band gap and the frontier molecular orbitals is not mutually exclusive. Consequently, to address this dichotomy is to construct materials based on building blocks that inherently possess deep HOMO energy levels such that link with other functional groups does not alarmingly raise the effective HOMO energy levels of the ensuing molecule.12,134,4-Difluoro-4-borata-3a-azonia-4a-aza-s-indacene (BODIPY) dyes attract considerable interest owing to a unique combination of facile synthesis, stability, high absorption coefficient, and high photoluminescence efficiency,14–18 along with the spectroscopic and photophysical properties of BODIPYs can be fine-tuned by attachment of ancillary residues at the appropriate positions of the BODIPY core.19,20 The BODIPY derivatives commonly possess solution-state absorption spectra with absorption maxima exceeding 500 nm, even up to 1000 nm, by a suitable modification.20–23 In addition, BODIPY inherently bear deep HOMO energy levels (<5.2 eV).19 Therefore, the relatively high extinction coefficient of the BODIPY core combined with deep HOMO energy levels and propensity to π-stack in solid-state, make BODIPY-based materials attractive candidates for organic electronic applications such as light-emitting diodes and organic photovoltaic.18,19,24,25
Inspired by the strategy for constructing low-band gap materials with conjugated donor–acceptor (D–A) system,1 herein, the highly flexible BODIPY platform was selected for the development of D–A–D type small molecular-based panchromatic light-harvester. In this paper, we designed five novel dyes incorporating uncommon reported meso-thiophene BODIPY26,27 as acceptor and different donors, including thiophene, bithiophene, carbazole, fluorine and triphenylamine, in the main backbone. We expect that these dyes would significant broadening of absorption and improving light-harvesting ability in the whole sunlight region. The photophysical properties and electrochemical behaviors of these dyes were studied systematically to reveal the relationships between the structures and the photoelectric characteristics. DFT theoretical calculations were performed to further understanding their structural and electronic features. Moreover, the photovoltaic performance was investigated to insight the potential applications of this kind of materials.
Results and discussion
Synthesis and characterization
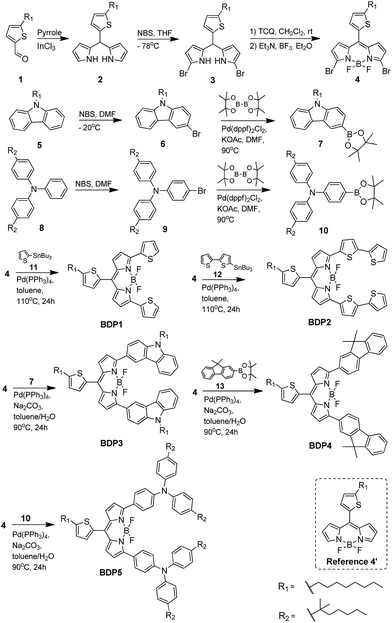
For the construction of these novel D–A–D type panchromatic BODIPY dyes, we utilized the Suzuki–Miyaura and Stille cross-coupling reaction to connect two electron-donor moieties covalently to the 3,5-positions of meso-thiophene BODIPY nucleus, where a substituted dibromated BODIPY 4 should be prerequisite for the synthesis (Scheme 1). Thus, our synthetic approach started with the preparation of 4 from commercially available 5-octylthiophene-2-carbaldehyde through a four-step sequence involving condensation–bisbromination–oxidation–chelation reaction. The dipyrromethane 2 was prepared in a mild way by InCl3 catalysis aldehyde 1 condensation with pyrrole at room temperature, which has been reported by our group.28 Subsequently, bisbromination of 2 with NBS under −78 °C afforded compound 3 in 65% yield. Oxidation of 3 with 2,3,5,6-tetrachloro-1,4-benzoquinone (TCQ) and then treated with BF3Et2O to form the important intermediate BODIPY 4. This reaction sequence turns out to be mild and efficient, and the key intermediate 4 was obtained with high quality. On the other hand, the important electron-donor segments were prepared according to the literature via the brominate-Miyaura coupling synthetic route. The corresponding borates such as compounds 7 and 10 were readily afforded though this strategy. Finally, the conversion from intermediate 4 to the desired BODIPY dyes (BDP1–5) was executed by the coupling 4 with the corresponding donor moieties via Suzuki and Stille reaction. To be noted that all these intermediates and the final BODIPY conjugates BDP1–5 were readily available, and the success in the synthesis of these products was characterized by a range of spectroscopies including 1H NMR, 13C NMR and MALDI-TOF-MS. Moreover, the target BODIPY dyes BDP1–5 were further accurately confirmed by HRMS.Electronic absorption
UV-Vis absorption and normalized absorption spectra of these BODIPY conjugates BDP1–5 in dichloromethane solution (2 × 10−5 mol L−1) are shown in Fig. 1 and 1 in ESI,† and the corresponding optical data are summarized in Table 1. It is a known fact that most of BODIPY's modification and functionalization have been undertaken at 1–3 and 5–7 positions, while the meso-position (8-position) has not been exploited fully in the π-extension of BODIPYs. The ever reported π-extended BODIPY chromophores have intrinsically two extinct intensive absorption bands,18 but there is a lack of significant absorption in the spectral region between these two features, which is harmful for the light-harvesting materials. Interestingly, this disadvantage can be compensated by fine-tune the meso-motifs of BODIPY. As shown in Fig. 1 and Scheme 1, meso-thiophene substituted BODIPY (4 and reference 4′) show two absorption bands. The more energetic one range of 400–500 nm can efficiently compensate the weak absorption range. Benefit for this feature, all these covalently assembled light harvesters (BDP1–5) showed panchromatic absorption with three major absorption bands (Fig. 1). The first one located at 250–400 nm due to the π–π*, S0–S1 transition derived from donor units, overlap with the S0–S2 of BODIPY.29 The second band range from 400 to 550 nm correspond to the π–π*, S0–S1 transition originated from meso-thiophene. The third band locate at long wavelength regions of 520–780 nm, peak at 628, 696, 623, 601 and 670 nm, respectively, is attributed to the intensive intramolecular charge transfer (ICT) interactions between two donor moieties and BODIPY core overlap with the ICT interaction originate from meso-thiophene to BODIPY core. Compared to the reference 4′, all these dyes with different substituents significantly broaden the absorption spectra and shift the absorption onsets to longer wavelength (Fig. 1). As we can see, BDP2 and BDP5 showed more bathochromic-shift than other ones. This is likely due to the dithiophene and triphenylamine moieties possess stronger electron donating ability and their more excellent molecular configuration, which facilitate π-conjugation electronic transfer between the D–A units. On the other hand, all these dyes showed high extinction coefficient range from 4.2 × 104 to 1.4 × 105 cm−1 M−1 in solution-state, which ensured their excellent light harvesting ability.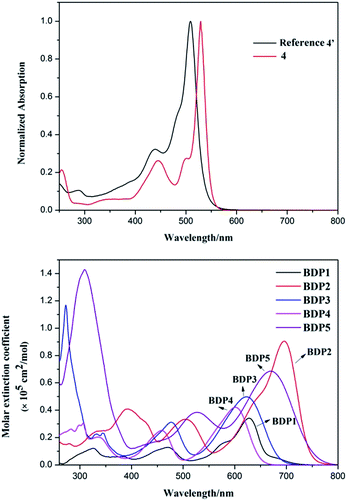 | ||
| Fig. 1 Normalized absorption spectra of the key intermediate 4 (above) and absorption spectra of BODIPY dyes BDP1–5 (below). | ||
| Entry | Solutiona | Filmb | |||||
|---|---|---|---|---|---|---|---|
| λmax (nm) | lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε ε |
λonset (nm) | Egc (eV) | λmax (nm) | λonset (nm) | Egc (eV) | |
| a Recorded in CH2Cl2.b Deposited onto quartz substrate by the spin-coating technique from CH2Cl2 solution.c Eg = 1240/λonset. | |||||||
| BDP1 | 326, 471, 628 | 4.09, 4.11, 4.63 | 750 | 1.65 | 328, 474, 654 | 850 | 1.46 |
| BDP2 | 392, 507, 696 | 4.61, 4.52, 4.96 | 780 | 1.59 | 424, 532, 752 | 950 | 1.31 |
| BDP3 | 273, 478, 623 | 5.07, 4.50, 4.70 | 710 | 1.71 | 273, 488, 653 | 802 | 1.55 |
| BDP4 | 305, 459, 601 | 4.48, 4.40, 4.62 | 685 | 1.81 | 307, 466, 625 | 870 | 1.43 |
| BDP5 | 308, 526, 670 | 5.16, 4.72, 4.83 | 782 | 1.59 | 305, 533, 681 | 800 | 1.55 |
In comparison with the absorption spectrum in CH2Cl2 solution, broader absorption bands with obvious bathochromic shifts were observed in the solid state (Fig. 2). This could be attributed to the molecular stacking in solid state, which suggests an increased conjugation length in the solid state due to the π–π stacking of the nearly planar conformations of BODIPY. Interestingly, BDP1 and BDP2 not only show broader absorption bands and bathochromic shifts but also exhibit a remarkable increase of the extinction coefficient (Fig. 2 and 2 in ESI†). It could be explain as BDP1-2 attachment with thiophene and dithiophene have the ability to form short S⋯S contact, which result in better intermolecular interactions and further increase conjugation length in solid.
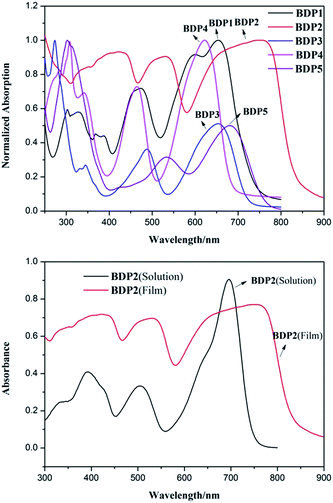 | ||
| Fig. 2 Normalized absorption spectra of dyes BDP1–5 in film (above) and the comparison of the absorption spectrum of BDP2 in solution state and in film state (below). | ||
It should be noted that all these dyes displayed perfect solubility and film-forming ability. The desirable combination of attractive photophysical characteristics, good solubility, favorable film-forming ability and functional group tolerance indicate that these dyes are promising applying in solution-processed organic electron devices such as light-harvesting antennae, BHJ photovoltaic solar cells, and so on.
Photoluminescence
The photoluminescence (PL) of complexes BDP1–5 in different solvents were investigated at room temperature. Rhodamine B (=0.71) was used as standard in methanol to estimate the fluorescence quantum yield.30 Their normalized emission spectra in CH2Cl2 solution at a concentration of 1 × 10−7 mol L−1 are illustrated in Fig. 3. The emission band maxima and quantum yields are listed in Table 2. Excitations of these compounds at their two absorption bands maximum corresponding to donor units and BODIPY units, resulted in the same near-infrared and deep red emissive BODIPY dyes with emission maxima of 644, 716, 686, 648 and 801 nm, respectively. This can be explained on the basis of intramolecular energy transfer from the BODIPY π–π* excited state to the lower lying singlet excited state of the donor moieties and/or to the existence of new non-radiative pathways from the BODIPY π–π* excited state to the ground state. These BODIPY dyes with donor units at 3,5-positions (BDP1–5) present a red-shift emission compared to the reference 4′, which indicate that the substituents significantly affect the emission and an effective intramolecular energy transfer for the excitons in the D–A units is occurred. Moreover, remarkable Stokes shifts of these dyes range from 480 to 2686 cm−1 were observed. Taking these features into account, we can assign the observed emission to the ICT emission. Due to the ICT process, BDP3 and BDP5 exhibit more significant bathochromic shift emissions than other BDPs, caused by the carbazole and triphenylamine substituents with stronger electron-donating ability. On the other hand, BDP1–5 gave low fluorescent quantum yield. These could be attributed to the increased internal conversion according to the energy gap law that states the non-radiative deactivation probability of S0–S1 increases as the energy gap of S0–S1 decreases in a highly extended conjugating system. Photoluminescence (PL) quenching provides the direct evidence for exciton dissociation, and thus efficient PL quenching is necessary to obtain efficient organic solar cells.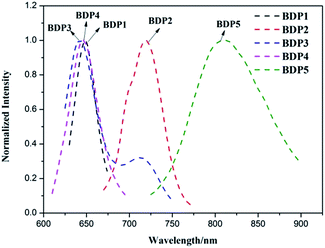 | ||
| Fig. 3 Normalized fluorescence spectra of BDP1–5 recorded in CH2Cl2 solution (c = 1 × 10−7 mol L−1). | ||
| Entry | λmax (nm) | Stocks shift (cm−1) | ϕPL | |||
|---|---|---|---|---|---|---|
| CH2Cl2 | Et2OAc | CH3COH3 | CH2Cl2 | |||
| BDP1 | 648 | 645 | 644 | 491 | 0.06 | |
| BDP2 | 720 | 714 | 716 | 480 | 0.04 | |
| BDP3 | 715 | 671 | 686 | 2065 | 0.05 | |
| BDP4 | 646 | 642 | 648 | 1160 | 0.12 | |
| BDP5 | 817 | 770 | 801 | 2686 | 0.04 | |
Solvent dependence study was performed in solvents with various polarities, such as dichloromethane, acetone and ethyl acetate (Table 2 and Fig. 3 in ESI†). The minor solvatochromic effect observed for BDP1-2 and BDP4 indicate that these emissions were not caused by solvent effect and/or aggregation effect. The results further confirmed the ICT emission features of these dyes. To be noted that the emission bands of the dyes BDP3 and BDP5 bathochromically shift to longer wavelengths in more polar solvents.
The drastic positive solvatochromic effect observed should be attributed to the N contained electron-donating groups (carbazole and triphenylamine), which enhance electron transfer from the symmetrical peripheral chromophores to the BODIPY motif.
Electrochemical properties
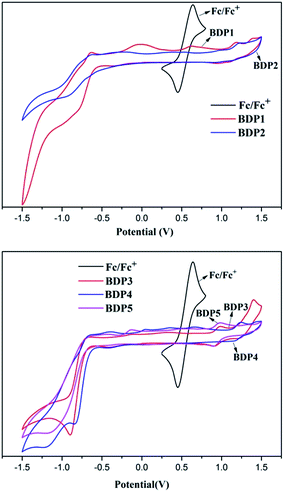
In order to investigate the electrochemical behaviors of BDP1–5, the electronic states of these dyes were studied through cyclic voltammetry in CH2Cl2 solution at a scan rate of 100 mV s−1 using n-Bu4NPF6 as supporting electrolyte. Potentials were standardized with ferrocene/ferrocenium (Fc/Fc+) couple as internal reference vs. Ag/AgCl. The cyclic voltammograms of BDP1–5 are illustrated in Fig. 4, and their electrochemical properties are summarized in Table 3. As shown in Fig. 4, dyes BDP1, BDP2 and BDP4 present reversible onset oxidation potential around 1.1 V, while BDP3 and BDP5 exhibit reversible onset oxidation potential around 0.9 V. It indicated that the aromatic substituents have obvious effects on the Eoxonset. The reduction potentials (Ered) calculated from Eoxonset − Eg were approximately matched with the experimental values obtained from reduction waves. The corresponding highest occupied molecular orbital (HOMO) energy level of BDP1–5 were calculated (5.36, 5.40, 5.15, 5.41 and 5.17 eV, respectively) from the equation EHOMO = [(−Eoxonset − 0.52)4.8] eV, where 0.52 V is the value for ferrocene versus Ag/AgCl and 4.8 eV is the energy level of Fc/Fc+ relative to the vacuum energy level. These dyes were actually located at very low energy level, which is attractive property in bulk-heterojunction (BHJ) solar cell device. The important factor for this result was that the strong electron donating group would cause an intensive ICT process. By neglecting any entropy change during light absorption, the reduction potential versus NHE (Ered), which corresponds to the lowest unoccupied molecular orbital (LUMO versus NHE), can be obtained from Eoxonset and Eg, where Eg is estimated from the absorption thresholds from absorption spectra in solution. The results were shown in Table 3. It should be noted that HOMO/LUMO levels of these dyes are match with the p-type semiconductor such as PCBM well, which suggest that they are capable of organic BHJ solar cells applications.| Entry | Ega (eV) | Eoxonsetb (eV) | Eoxpc (v) | Eredd (v) | HOMO (eV) | LUMOe (eV) |
|---|---|---|---|---|---|---|
| a Eg, estimated from the absorption spectra of dyes absorbed in CH2Cl2 solution, Eg = 1240/λonset.b Eoxonset, onset oxidation potential.c Eoxp, oxidation peak potential.d Ered, the reduction potential, was calculated from Eoxonset − Eg.e EHOMO = [(−Eoxonset − 0.52)4.8] eV, ELUMO = EHOMO + Eg eV. | ||||||
| BDP1 | 1.65 | 1.10 | 1.18 | −0.47 | −5.36 | −3.81 |
| BDP2 | 1.59 | 1.14 | 1.23 | −0.45 | −5.40 | −3.81 |
| BDP3 | 1.69 | 0.89 | 0.98 | −0.80 | −5.15 | −3.46 |
| BDP4 | 1.81 | 1.15 | 1.24 | −0.66 | −5.41 | −3.60 |
| BDP5 | 1.59 | 0.91 | 0.99 | −0.68 | −5.17 | −3.58 |
Theoretical calculations
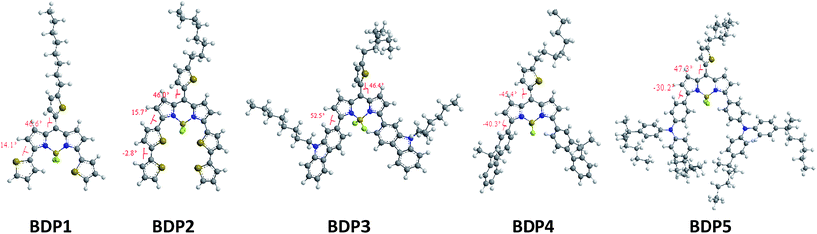
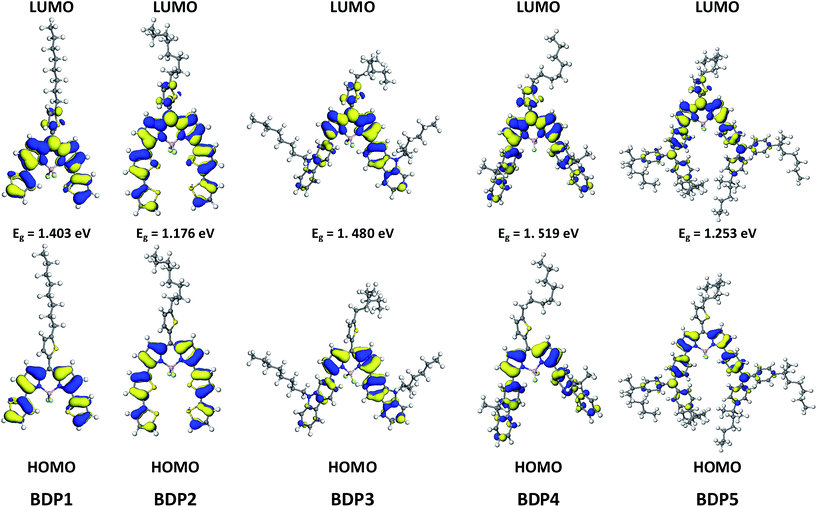
For a better understanding of the structural and electronic features of these novel dyes, the DFT theoretical calculations were further performed, and the optimized structures and the electronic distribution in HOMO and LUMO levels are presented in Fig. 5 and 6. All calculations were carried out with the Gaussian 09 program suite by using the B3LYP method and 6-31 G* basis set. From the optimized ground-state geometries of the dyes in Fig. 5, we can see that the dihedral angle between the donor units and the BODIPY framework are computed to be 14.1°, 15.7°, 52.5°, 40.3° and 30.2°, respectively, for BDP1–5. This calculated results indicated that the BDP1 and BDP2 produce a higher degree of molecular coplanarity than BDP3 and BDP4, which is in accordance with the absorption spectra observed in solution. While the noticeable bathochromic shift in BDP5 could be attributed to a π–π* mixed twisted intramolecular charge transfer (TICT) transition originated from the appended strong electron-donating triphenylamine group to the central BODIPY component.31 The dihedral angle between meso-thiophene and BODIPY core are around 46°, which amenable to further extending π-conjugation as observed in Fig. 1.The electron distributions of BDP1–5 indicated that π-electrons in the HOMO are delocalized over the entire molecule backbone, offering effective orbital interactions among the stacked π-systems, while their LUMOs are mainly localized on the BODIPY moieties. Therefore, the HOMO–LUMO transition that is the dominant contributing configuration to the S1 state in BDP1–5 should be ascribed to a mixture of π–π* and ICT characters, which is consistent with their absorption assignments. It should be noted that their LUMOs were also locate at the meso-thiophene motif, which could not been seen in commonly reported meso-phenyl BODIPY derivatives,32,33 indicate that effective π-conjugation was formed between the BODIPY framework and meso-thiophene unit. The extended π-conjugation is helpful for broadening absorption response ranges, and this have be attested by the UV-Vis absorption. On the other hand, the calculated results clearly demonstrate that strong electron-donating substituent with more coplanar geometry, such as dithiophene in BDP2, increases the energy level of HOMO more than that of LUMO, thus the HOMO–LUMO gap decreases, causing a bathochromic shift of the π–π*/ICT band. This trend follows that observed from the UV-Vis absorption measurement. Fig. 6 presented the HOMO–LUMO energy differences (energy band gaps, calculated Ecalg) of BDP1–5. We can see that the trend of the predicted Ecalg values (1.18–1.52 eV) is in consistent with the estimated Eoptg (calculated from the ground-state absorption, 1.59–1.81 eV) in Table 1.
Photovoltaic performance
In recent years, small molecule-based organic OPV attract desirable attention due to their special advantages.34–41 BODIPY derivatives are promising compounds to be used in the active layer of OPV devices.18,25 However, the examples were rather limited. It is imperative to develop novel structures based on BODIPY for OPV investigations. The above discussed results indicate that the photophysical and electrochemical properties of dyes BDP1–5 were excellent and their performance as electron donor in BHJ solar cells would be feasible.We expect that they would become valuable candidates to the application of BHJ solar cells as a series of new organic small molecule-based dyes. Herein, BDP2 was chosen to construct BHJ solar cell device for insight the potential applications of this kind of materials.
The BHJ solar cell device was fabricated using BDP2 as the electron donor material and PC61BM as the electron acceptor material through the conventional spin-coating process from CHCl3 solution with a concentration of 15 mg mL−1 at room temperature. The cell structure was ITO/PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS (30 nm)/donor
PSS (30 nm)/donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor (1
acceptor (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 100 nm)/LiF (0.7 nm)/Al (100 nm). The effective device area was 1 × 10−2 cm2, as defined by a shadow mask. Fig. 7 shows typical current density–voltage (J–V) characteristic of optimized device at a ratio of 1
3, 100 nm)/LiF (0.7 nm)/Al (100 nm). The effective device area was 1 × 10−2 cm2, as defined by a shadow mask. Fig. 7 shows typical current density–voltage (J–V) characteristic of optimized device at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
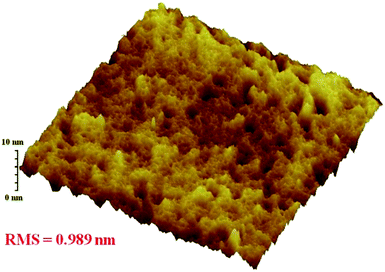
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 between BDP2 and PC61BM under 1000 W m−2 air mass 1.5 global (AM 1.5 G) illumination. Device parameters such as short-circuit photocurrent density (Jsc), open-circuit voltage (Voc), fill factor (FF), and solar energy-to-electricity conversion yields (PCE) were deduced from the J–V characteristics and summarized in Table 4. A PCE of 2.12% with a Voc of 0.73 V, a Jsc of 7.64 mA cm2, and a FF of 38% was achieved in the BDP2: PC61BM-based device. To be noted that the PCE of BHJ solar cell was obtained without any thermal annealing or high boiling temperature additive in the solutions. As we expected, the device based on BDP2 afford relatively higher Jsc value, higher than most of analogous device based on other BODIPY derivatives,19,25 which is mainly attributed to the panchromatic effective absorption. However, FF value of this device is relatively low, which suggest that the charge transfer within the cell was hindered once the charges in the active layer are formed.4,42 To further get some information, the morphology of blend film of BDP2/PC61BM was tested using atomic force microscopy (AFM), and is shown in Fig. 8. The film surface was relative flat with a root-mean-square (RMS) roughness of 0.989 nm, and relative small phase domains were observed. This indicated that BDP2 have a good miscibility with PC61BM, while fail to form a well-interpenetrating network with a suitable phase separation scale in device, which lead to the low FF. Further optimization of device structure and fabrication process and determination the photovoltaic performances of other dyes (BDP1 and BDP3–5) were undergoing.
3 between BDP2 and PC61BM under 1000 W m−2 air mass 1.5 global (AM 1.5 G) illumination. Device parameters such as short-circuit photocurrent density (Jsc), open-circuit voltage (Voc), fill factor (FF), and solar energy-to-electricity conversion yields (PCE) were deduced from the J–V characteristics and summarized in Table 4. A PCE of 2.12% with a Voc of 0.73 V, a Jsc of 7.64 mA cm2, and a FF of 38% was achieved in the BDP2: PC61BM-based device. To be noted that the PCE of BHJ solar cell was obtained without any thermal annealing or high boiling temperature additive in the solutions. As we expected, the device based on BDP2 afford relatively higher Jsc value, higher than most of analogous device based on other BODIPY derivatives,19,25 which is mainly attributed to the panchromatic effective absorption. However, FF value of this device is relatively low, which suggest that the charge transfer within the cell was hindered once the charges in the active layer are formed.4,42 To further get some information, the morphology of blend film of BDP2/PC61BM was tested using atomic force microscopy (AFM), and is shown in Fig. 8. The film surface was relative flat with a root-mean-square (RMS) roughness of 0.989 nm, and relative small phase domains were observed. This indicated that BDP2 have a good miscibility with PC61BM, while fail to form a well-interpenetrating network with a suitable phase separation scale in device, which lead to the low FF. Further optimization of device structure and fabrication process and determination the photovoltaic performances of other dyes (BDP1 and BDP3–5) were undergoing.
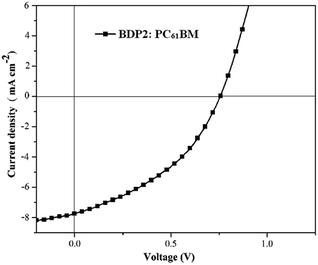 | ||
Fig. 7 Current density–voltage (J–V) characteristics of the device at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 weight ratio between BDP2 and PC61BM. 3 weight ratio between BDP2 and PC61BM. | ||
| Donor | Acceptor | Jsc (mA cm−2) | Voc (V) | FF | PCE % |
|---|---|---|---|---|---|
| BDP2 | PC61BM | 7.64 | 0.73 | 0.38 | 2.12 |
Experimental
Materials and characterization
Compound 5-octylthiophene-2-carbaldehyde (1), 9-octyl-9H-carbazole (5), 4-(2-methylheptan-2-yl)-N-(4-(2-methylheptan-2-yl)phenyl)-N-phenylaniline (8), tributyl(thiophen-2-yl)stannane (11), [2,2′-bithiophen]-5-yltributylstannane (12), 2-(9,9-dimethyl-9H-fluoren-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (13) were synthesized according to the literature procedures.43–45 THF and toluene were dried and distilled over sodium and benzophenone. Dichloromethane, ethyl acetate, methanol were dried under 4A molecular sieves. Pyrrole was distilled from CaH2 before use. All other chemicals with reagent-grade were purchased and used without further purification unless otherwise specified. Column chromatography was performed on silica (300–400 mesh). 1H and 13C NMR spectra were recorded on a Bruker Avance spectrometer (600 and 151 MHz, respectively) with tetramethylsilane (TMS) as the internal standard. Matrix-assisted laser desorption/ionization time of flight mass spectra (MALDI-TOF-MS) were recorded on Bruker ultraflex-II spectrometer, matrix-assisted laser desorption ionization mass spectrometry using an α-cyano-4-hydroxy-cinnamic acid (α-CHCA) matrix. High resolution mass spectra (HRMS) were recorded on an Agilent 6530 Accurate Mass Q-TOF LC-MS instrument. Absorption spectra were collected on an SHIMADZU UV-2550 UV-Vis spectrophotometer and emission spectra were recorded by using a Hitachi F-4500 instrument. Excitation and emission spectra were fully corrected by reference to a standard lamp. Cyclic voltammetric (CV) was carried out on a CHI660E electrochemical workstation utilizing the three electrode configuration consisting of a gold electrode (working electrode), platinum wire (auxiliary electrode) and Ag/AgCl electrode (reference electrode). The experiment was performed in dry dichloromethane solution using 0.1 M n-Bu4NPF6 as supporting electrolyte. The solution was deoxygenated at room temperature under nitrogen protection.Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent afforded 2 as an orange solid (1.2 g, yield 53%). 1H NMR (600 MHz, CDCl3) δ 7.74 (s, 2H), 6.54 (s, 1H), 6.53 (d, J = 2.8 Hz, 1H), 6.51 (s, 2H), 6.07 (s, 2H), 5.95 (s, 2H), 5.48 (s, 1H), 1.64–1.56 (m, 2H), 1.34–1.16 (m, 10H), 0.85 (t, J = 6.8 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 145.29, 142.93, 132.38, 125.14, 123.47, 117.52, 108.38, 107.12, 39.34, 32.01, 31.76, 30.36, 29.47, 29.38, 29.35, 22.83, 14.30.
1, v/v) as eluent afforded 2 as an orange solid (1.2 g, yield 53%). 1H NMR (600 MHz, CDCl3) δ 7.74 (s, 2H), 6.54 (s, 1H), 6.53 (d, J = 2.8 Hz, 1H), 6.51 (s, 2H), 6.07 (s, 2H), 5.95 (s, 2H), 5.48 (s, 1H), 1.64–1.56 (m, 2H), 1.34–1.16 (m, 10H), 0.85 (t, J = 6.8 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 145.29, 142.93, 132.38, 125.14, 123.47, 117.52, 108.38, 107.12, 39.34, 32.01, 31.76, 30.36, 29.47, 29.38, 29.35, 22.83, 14.30.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the desired compound 3 as brown solid (1.86 g, yield 65%). 1H NMR (600 MHz, CDCl3) δ 7.96 (s, 2H), 6.66 (d, J = 3.4 Hz, 1H), 6.61 (d, J = 3.3 Hz, 1H), 6.08 (d, J = 3.1 Hz, 2H), 5.95 (d, J = 3.0 Hz, 2H), 5.51 (s, 1H), 2.74 (t, J = 7.6 Hz, 2H), 1.67–1.59 (m, 2H), 1.36–1.23 (m, 10H), 0.88 (t, J = 6.9 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 146.00, 140.97, 132.84, 125.47, 123.58, 110.60, 109.02, 97.49, 39.46, 31.87, 31.56, 30.23, 29.32, 29.23, 29.20, 22.68, 14.14. MALDI-TOF-MS, m/z: calcd for C21H26Br2N2S [M]+: 498.016, found: 498.199.
1) to give the desired compound 3 as brown solid (1.86 g, yield 65%). 1H NMR (600 MHz, CDCl3) δ 7.96 (s, 2H), 6.66 (d, J = 3.4 Hz, 1H), 6.61 (d, J = 3.3 Hz, 1H), 6.08 (d, J = 3.1 Hz, 2H), 5.95 (d, J = 3.0 Hz, 2H), 5.51 (s, 1H), 2.74 (t, J = 7.6 Hz, 2H), 1.67–1.59 (m, 2H), 1.36–1.23 (m, 10H), 0.88 (t, J = 6.9 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 146.00, 140.97, 132.84, 125.47, 123.58, 110.60, 109.02, 97.49, 39.46, 31.87, 31.56, 30.23, 29.32, 29.23, 29.20, 22.68, 14.14. MALDI-TOF-MS, m/z: calcd for C21H26Br2N2S [M]+: 498.016, found: 498.199.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent, afforded the important intermediate 4 as red solid (820 mg, yield 75%). 1H NMR (600 MHz, CDCl3) δ 7.34 (d, J = 3.5 Hz, 1H), 7.18 (d, J = 4.1 Hz, 2H), 6.94 (d, J = 3.4 Hz, 1H), 6.56 (d, J = 4.2 Hz, 2H), 2.90 (t, J = 7.6 Hz, 2H), 1.76–1.71 (m, 2H), 1.44–1.24 (m, 10H), 0.89 (t, J = 6.8 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 154.09, 136.14, 134.71, 133.57, 131.53, 130.49, 125.91, 122.43, 31.84, 31.49, 30.42, 29.26, 29.18, 29.17, 22.66, 14.12. MALDI-TOF-MS, m/z: calcd for C21H23BBr2F2N2S [M]+: 543.999, found: 544.156.
1, v/v) as eluent, afforded the important intermediate 4 as red solid (820 mg, yield 75%). 1H NMR (600 MHz, CDCl3) δ 7.34 (d, J = 3.5 Hz, 1H), 7.18 (d, J = 4.1 Hz, 2H), 6.94 (d, J = 3.4 Hz, 1H), 6.56 (d, J = 4.2 Hz, 2H), 2.90 (t, J = 7.6 Hz, 2H), 1.76–1.71 (m, 2H), 1.44–1.24 (m, 10H), 0.89 (t, J = 6.8 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 154.09, 136.14, 134.71, 133.57, 131.53, 130.49, 125.91, 122.43, 31.84, 31.49, 30.42, 29.26, 29.18, 29.17, 22.66, 14.12. MALDI-TOF-MS, m/z: calcd for C21H23BBr2F2N2S [M]+: 543.999, found: 544.156.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent, afforded 6 as white solid (2.54 g, yield 71%). 1H NMR (600 MHz, CDCl3), δ: 8.21 (s, 1H), 8.05 (d, 1H), 7.53 (d, 1H), 7.48 (d, J = 7.2 Hz, 1H), 7.40 (d, 1H), 7.27 (t, J = 12.0 Hz, 2H), 4.26 (t, 2H), 1.84 (m, 2H), 1.25 (m, 10H), 0.85 (t, 3H).
1, v/v) as eluent, afforded 6 as white solid (2.54 g, yield 71%). 1H NMR (600 MHz, CDCl3), δ: 8.21 (s, 1H), 8.05 (d, 1H), 7.53 (d, 1H), 7.48 (d, J = 7.2 Hz, 1H), 7.40 (d, 1H), 7.27 (t, J = 12.0 Hz, 2H), 4.26 (t, 2H), 1.84 (m, 2H), 1.25 (m, 10H), 0.85 (t, 3H).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent, afforded 7 as white solid (1.7 g, yield 76%). 1H NMR (600 MHz, CDCl3), δ: 8.60 (s, 1H), 8.13 (d, J = 7.6 Hz, 1H), 7.92 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 7.3 Hz, 1H), 7.40 (t, J = 9.5 Hz, 2H), 7.23 (d, J = 7.0 Hz, 1H), 4.30 (t, J = 6.9 Hz, 2H), 1.90–1.86 (m, 2H), 1.44 (s, 12H), 1.36–1.28 (m, 10H), 0.85 (t, J = 13.6 Hz, 3H). MALDI-TOF-MS, m/z: calcd for C26H36BNO2 [M]+: 405.300, found 405.333.
1, v/v) as eluent, afforded 7 as white solid (1.7 g, yield 76%). 1H NMR (600 MHz, CDCl3), δ: 8.60 (s, 1H), 8.13 (d, J = 7.6 Hz, 1H), 7.92 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 7.3 Hz, 1H), 7.40 (t, J = 9.5 Hz, 2H), 7.23 (d, J = 7.0 Hz, 1H), 4.30 (t, J = 6.9 Hz, 2H), 1.90–1.86 (m, 2H), 1.44 (s, 12H), 1.36–1.28 (m, 10H), 0.85 (t, J = 13.6 Hz, 3H). MALDI-TOF-MS, m/z: calcd for C26H36BNO2 [M]+: 405.300, found 405.333.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent, afforded 9 as white solid (5.1 g, yield 93%). 1H NMR (600 MHz, CDCl3) δ 7.31 (d, J = 8.7 Hz, 2H), 7.27 (d, J = 8.5 Hz, 4H), 6.99 (d, J = 8.6 Hz, 4H), 6.92 (d, J = 8.9 Hz, 2H), 1.74 (s, 4H), 1.43–1.36 (m, 12H), 0.81–0.76 (m, 18H).
1, v/v) as eluent, afforded 9 as white solid (5.1 g, yield 93%). 1H NMR (600 MHz, CDCl3) δ 7.31 (d, J = 8.7 Hz, 2H), 7.27 (d, J = 8.5 Hz, 4H), 6.99 (d, J = 8.6 Hz, 4H), 6.92 (d, J = 8.9 Hz, 2H), 1.74 (s, 4H), 1.43–1.36 (m, 12H), 0.81–0.76 (m, 18H).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent, from which the desired product BDP1 was obtained as purple solid (81 mg, yield 40%). 1H NMR (600 MHz, CDCl3) δ 8.19 (d, J = 3.6 Hz, 2H), 7.48 (d, J = 5.0 Hz, 2H), 7.29 (d, J = 3.5 Hz, 1H), 7.22–7.16 (m, 4H), 6.92 (d, J = 3.5 Hz, 1H), 6.84 (d, J = 4.3 Hz, 2H), 2.91 (t, J = 6.0 Hz, 2H), 1.78–1.73 (m, 2H), 1.46–1.25 (m, 10H), 0.92 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 151.68, 149.81, 136.22, 134.40, 132.36, 132.09, 131.20, 131.14, 131.09, 130.16, 129.03, 125.12, 120.47, 33.66, 31.54, 30.33, 30.01, 29.72, 28.88, 22.58, 14.09. MALDI-TOF-MS, m/z: calcd for C29H29BF2N2S3 [M]+: 550.155, found: 550.360. HRMS, m/z: calcd for C29H29BF2N2S3 [M]+: 550.1554, found: 550.1566.
1, v/v) as eluent, from which the desired product BDP1 was obtained as purple solid (81 mg, yield 40%). 1H NMR (600 MHz, CDCl3) δ 8.19 (d, J = 3.6 Hz, 2H), 7.48 (d, J = 5.0 Hz, 2H), 7.29 (d, J = 3.5 Hz, 1H), 7.22–7.16 (m, 4H), 6.92 (d, J = 3.5 Hz, 1H), 6.84 (d, J = 4.3 Hz, 2H), 2.91 (t, J = 6.0 Hz, 2H), 1.78–1.73 (m, 2H), 1.46–1.25 (m, 10H), 0.92 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 151.68, 149.81, 136.22, 134.40, 132.36, 132.09, 131.20, 131.14, 131.09, 130.16, 129.03, 125.12, 120.47, 33.66, 31.54, 30.33, 30.01, 29.72, 28.88, 22.58, 14.09. MALDI-TOF-MS, m/z: calcd for C29H29BF2N2S3 [M]+: 550.155, found: 550.360. HRMS, m/z: calcd for C29H29BF2N2S3 [M]+: 550.1554, found: 550.1566.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent to give the pure compound BDP2 as purple solid in 52% yield. 1H NMR (600 MHz, CDCl3) δ 8.13 (d, J = 4.0 Hz, 2H), 7.31–7.26 (m, 7H), 7.17 (d, J = 4.3 Hz, 2H), 7.06–7.04 (m, 2H), 6.90 (d, J = 3.2 Hz, 1H), 6.83 (d, J = 4.3 Hz, 2H), 2.90 (t = 6.5, 2H), 1.78–1.72 (m, 2H), 1.45–1.24 (m, 10H), 0.98 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 151.52, 148.89, 140.99, 136.89, 136.72, 136.66, 133.02, 132.44, 132.18, 131.97, 129.91, 128.12, 125.82, 125.53, 125.10, 124.70, 120.41, 33.67, 31.55, 30.34, 30.02, 28.90, 28.29, 22.59, 14.10. MALDI-TOF-MS, m/z: calcd for C37H33BF2N2S5 [M − F]+: 695.131, found: 695.312. HRMS, m/z: calcd for C37H33BF2N2S5 [M]+: 714.1308, found: 714.1321.
1, v/v) as eluent to give the pure compound BDP2 as purple solid in 52% yield. 1H NMR (600 MHz, CDCl3) δ 8.13 (d, J = 4.0 Hz, 2H), 7.31–7.26 (m, 7H), 7.17 (d, J = 4.3 Hz, 2H), 7.06–7.04 (m, 2H), 6.90 (d, J = 3.2 Hz, 1H), 6.83 (d, J = 4.3 Hz, 2H), 2.90 (t = 6.5, 2H), 1.78–1.72 (m, 2H), 1.45–1.24 (m, 10H), 0.98 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 151.52, 148.89, 140.99, 136.89, 136.72, 136.66, 133.02, 132.44, 132.18, 131.97, 129.91, 128.12, 125.82, 125.53, 125.10, 124.70, 120.41, 33.67, 31.55, 30.34, 30.02, 28.90, 28.29, 22.59, 14.10. MALDI-TOF-MS, m/z: calcd for C37H33BF2N2S5 [M − F]+: 695.131, found: 695.312. HRMS, m/z: calcd for C37H33BF2N2S5 [M]+: 714.1308, found: 714.1321.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent, afforded the desired product BDP3 as purple solid (150 mg, yield 41%). 1H NMR (600 MHz, CDCl3) δ 8.70 (s, 2H), 8.14 (d, J = 7.5 Hz, 2H), 8.07 (d, J = 8.6 Hz, 2H), 7.92 (d, J = 8.3 Hz, 1H), 7.46–7.42 (m, 2H), 7.40–7.35 (m, 4H), 7.30 (d, J = 4.2 Hz, 2H), 7.21 (d, J = 7.7 Hz, 2H), 6.94 (d, J = 3.2 Hz, 1H), 6.79 (d, J = 4.2 Hz, 2H), 4.24 (t, J = 7.3 Hz, 4H), 2.94 (t, J = 7.6 Hz, 2H), 1.88–1.82 (m, 4H), 1.80–1.76 (m, 2H), 1.36–1.19 (m, 30H), 1.01 (t, J = 7.4 Hz, 3H), 0.88–0.83 (m, 6H). 13C NMR (151 MHz, CDCl3) δ 159.10, 142.61, 141.09, 140.91, 132.09, 130.07, 127.69, 125.78, 125.60, 123.67, 123.24, 122.84, 122.06, 120.69, 120.59, 119.22, 119.18, 108.80, 108.74, 108.43, 108.10, 83.59, 43.24, 43.12, 31.80, 29.39, 29.36, 29.18, 29.02, 28.95, 27.33, 22.61, 14.09. MALDI-TOF-MS, m/z: calcd for C61H71BF2N4S [M]+: 940.546, found: 940.631. HRMS, m/z: calcd for C61H71BF2N4S [M]+: 940.5461, found: 940.5457.
1, v/v) as eluent, afforded the desired product BDP3 as purple solid (150 mg, yield 41%). 1H NMR (600 MHz, CDCl3) δ 8.70 (s, 2H), 8.14 (d, J = 7.5 Hz, 2H), 8.07 (d, J = 8.6 Hz, 2H), 7.92 (d, J = 8.3 Hz, 1H), 7.46–7.42 (m, 2H), 7.40–7.35 (m, 4H), 7.30 (d, J = 4.2 Hz, 2H), 7.21 (d, J = 7.7 Hz, 2H), 6.94 (d, J = 3.2 Hz, 1H), 6.79 (d, J = 4.2 Hz, 2H), 4.24 (t, J = 7.3 Hz, 4H), 2.94 (t, J = 7.6 Hz, 2H), 1.88–1.82 (m, 4H), 1.80–1.76 (m, 2H), 1.36–1.19 (m, 30H), 1.01 (t, J = 7.4 Hz, 3H), 0.88–0.83 (m, 6H). 13C NMR (151 MHz, CDCl3) δ 159.10, 142.61, 141.09, 140.91, 132.09, 130.07, 127.69, 125.78, 125.60, 123.67, 123.24, 122.84, 122.06, 120.69, 120.59, 119.22, 119.18, 108.80, 108.74, 108.43, 108.10, 83.59, 43.24, 43.12, 31.80, 29.39, 29.36, 29.18, 29.02, 28.95, 27.33, 22.61, 14.09. MALDI-TOF-MS, m/z: calcd for C61H71BF2N4S [M]+: 940.546, found: 940.631. HRMS, m/z: calcd for C61H71BF2N4S [M]+: 940.5461, found: 940.5457.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent to give the pure compound BDP4 as purple solid in 58% yield. 1H NMR (600 MHz, CDCl3) δ 8.02 (s, 2H), 7.89 (d, J = 9.2 Hz, 2H), 7.75–7.71 (m, 4H), 7.41 (d, J = 8.1 Hz, 2H), 7.39 (d, J = 3.5 Hz, 1H), 7.34–7.29 (m, 6H), 6.96 (d, J = 3.4 Hz, 1H), 6.75 (d, J = 4.2 Hz, 2H), 2.98–2.91 (m, 2H), 1.81–1.75 (m, 2H), 1.48 (s, 12H), 1.39–1.23 (m, 10H), 1.01 (t, J = 7.3 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 158.62, 154.41, 153.32, 151.87, 140.51, 138.70, 136.05, 133.89, 132.45, 131.65, 130.57, 128.72, 127.67, 127.00, 125.20, 124.02, 122.60, 120.79, 120.42, 119.65, 83.75, 46.95, 33.73, 30.06, 29.77, 29.69, 29.35, 27.08, 24.93, 22.32, 13.85. MALDI-TOF-MS, m/z: calcd for C51H49BF2N2S [M]+: 770.368, found: 770.479. HRMS, m/z: calcd for C51H49BF2N2S [M]+: 770.3678, found: 770.3691.
1, v/v) as eluent to give the pure compound BDP4 as purple solid in 58% yield. 1H NMR (600 MHz, CDCl3) δ 8.02 (s, 2H), 7.89 (d, J = 9.2 Hz, 2H), 7.75–7.71 (m, 4H), 7.41 (d, J = 8.1 Hz, 2H), 7.39 (d, J = 3.5 Hz, 1H), 7.34–7.29 (m, 6H), 6.96 (d, J = 3.4 Hz, 1H), 6.75 (d, J = 4.2 Hz, 2H), 2.98–2.91 (m, 2H), 1.81–1.75 (m, 2H), 1.48 (s, 12H), 1.39–1.23 (m, 10H), 1.01 (t, J = 7.3 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 158.62, 154.41, 153.32, 151.87, 140.51, 138.70, 136.05, 133.89, 132.45, 131.65, 130.57, 128.72, 127.67, 127.00, 125.20, 124.02, 122.60, 120.79, 120.42, 119.65, 83.75, 46.95, 33.73, 30.06, 29.77, 29.69, 29.35, 27.08, 24.93, 22.32, 13.85. MALDI-TOF-MS, m/z: calcd for C51H49BF2N2S [M]+: 770.368, found: 770.479. HRMS, m/z: calcd for C51H49BF2N2S [M]+: 770.3678, found: 770.3691.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent to give the pure compound BDP5 as purple solid in 45% yield. 1H NMR (600 MHz, CDCl3) δ 7.78 (d, J = 8.8 Hz, 4H), 7.62 (d, J = 8.4 Hz, 4H), 7.44 (d, J = 8.7 Hz, 1H), 7.24 (d, J = 8.6 Hz, 8H), 7.18 (d, J = 4.3 Hz, 2H), 7.07 (d, J = 8.6 Hz, 8H), 6.89 (d, J = 3.5 Hz, 1H), 6.65 (d, J = 4.3 Hz, 2H), 2.92–2.89 (m, 2H), 1.83 (d, J = 8.6 Hz, 2H), 1.71 (d, J = 11.8 Hz, 8H), 1.37–1.25 (m, 34H), 0.98 (t, J = 7.4 Hz, 3H), 0.78–0.73 (m, 36H). 13C NMR (151 MHz, CDCl3) δ 151.04, 149.14, 145.67, 145.10, 144.48, 144.07, 135.70, 130.53, 127.13, 127.08, 127.02, 126.18, 125.15, 124.96, 124.56, 124.46, 124.36, 123.54, 120.90, 119.97, 57.21, 57.18, 38.28, 38.21, 32.45, 31.79, 31.72, 31.44, 29.96, 29.69, 29.37, 29.25, 27.19, 24.87, 22.34, 14.14. MALDI-TOF-MS, m/z: calcd for C89H115BF2N4S [M]+: 1320.890, found: 1321.053. HRMS, m/z: calcd for C89H115BF2N4S [M]+: 1320.8904, found: 1320.8911.
1, v/v) as eluent to give the pure compound BDP5 as purple solid in 45% yield. 1H NMR (600 MHz, CDCl3) δ 7.78 (d, J = 8.8 Hz, 4H), 7.62 (d, J = 8.4 Hz, 4H), 7.44 (d, J = 8.7 Hz, 1H), 7.24 (d, J = 8.6 Hz, 8H), 7.18 (d, J = 4.3 Hz, 2H), 7.07 (d, J = 8.6 Hz, 8H), 6.89 (d, J = 3.5 Hz, 1H), 6.65 (d, J = 4.3 Hz, 2H), 2.92–2.89 (m, 2H), 1.83 (d, J = 8.6 Hz, 2H), 1.71 (d, J = 11.8 Hz, 8H), 1.37–1.25 (m, 34H), 0.98 (t, J = 7.4 Hz, 3H), 0.78–0.73 (m, 36H). 13C NMR (151 MHz, CDCl3) δ 151.04, 149.14, 145.67, 145.10, 144.48, 144.07, 135.70, 130.53, 127.13, 127.08, 127.02, 126.18, 125.15, 124.96, 124.56, 124.46, 124.36, 123.54, 120.90, 119.97, 57.21, 57.18, 38.28, 38.21, 32.45, 31.79, 31.72, 31.44, 29.96, 29.69, 29.37, 29.25, 27.19, 24.87, 22.34, 14.14. MALDI-TOF-MS, m/z: calcd for C89H115BF2N4S [M]+: 1320.890, found: 1321.053. HRMS, m/z: calcd for C89H115BF2N4S [M]+: 1320.8904, found: 1320.8911.Conclusions
In summary, we have successfully designed and synthesized a series of novel structurally constrained meso-thiophene BODIPY dyes (BDP1–5) with a variety of electron-donating substituents at 3,5-positions. These dyes exhibit excellent panchromatic absorption with high extinction coefficients both in solution and in film, especially, BDP1-2 dramatically promote the ε values in film state, which benefit them harvesting the whole range of solar light. Additionally, all these light-harvesters show good solubility and perfect film-forming ability, which are attractive factors for solution-processed organic film devices. Luminescence of these dyes located at near-infrared and the red regions with relative low fluorescent quantum yields. The HOMO/LUMO energy levels investigated by CV were about 5.4 eV and 3.8 eV, which are fascinating for BHJ donor materials. The photovoltaic device based on BDP2 and PC61BM exhibit a potential power conversion efficiency of 2.12%, a moderate open-circuit voltage of 0.73 V and a relative high short-circuit photocurrent density of 7.64 mA cm−2.Acknowledgements
We greatly acknowledge the financial support from the Nature Science Foundation of Guangdong Province (2014A030313630), the National Nature Science Foundation of China (51476036, 21342003), Project for High-level Personnel of University in Guangdong Province ([2013]246), the Production-Study-Research Combinational Project of Guangdong Province and Ministry of Education (2012B091100296), the University Science and Technology Innovation Key Project of Guangdong Province (cxzd1148) and the University and Scientific Research Institution Key Project of Dongguan City (2012108101005).Notes and references
- Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef CAS PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS PubMed.
- C. L. Chochos and S. A. Choulis, Prog. Polym. Sci., 2011, 36, 1326 CrossRef CAS PubMed.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153 CrossRef CAS PubMed.
- L. Dou, J. You, Z. Hong, Z. Xu, G. Li, R. A. Street and Y. Yang, Adv. Mater., 2013, 25, 6642 CrossRef CAS PubMed.
- L. Dou, J. You, J. Yang, C. Chen, Y. He, S. Murase, T. Moriarty, K. Emery, G. Li and Y. Yang, Nat. Photonics, 2012, 6, 180 CrossRef CAS PubMed.
- Y. Lin, Y. Li and X. Zhan, Chem. Soc. Rev., 2012, 41, 4245 RSC.
- C. P. Lee, R. Y.-Y. Lin, L. Y. Lin, C.-T. Li, T.-C. Chu, S.-S. Sun, J. T. Lin and K.-C. Ho, RSC Adv., 2015, 5, 23810 RSC.
- L. Beverina, R. Ruffo, M. M. Salamone, E. Ronchi, M. Binda, D. Natali and M. Sampietro, J. Mater. Chem., 2012, 22, 6704 RSC.
- B. Kippelen and J.-L. Bredas, Energy Environ. Sci., 2009, 2, 251 CAS.
- M. C. Scharber, D. Muhlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789 CrossRef CAS PubMed.
- H. Zhou, L. Yang and W. You, Macromolecules, 2012, 45, 607 CrossRef CAS.
- H. Zhou, L. Yang, S. C. Price, K. J. Knight and W. You, Angew. Chem., Int. Ed., 2010, 49, 7992 CrossRef CAS PubMed.
- F. Marsico, A. Turshatov, K. Weber and F. R. Wurm, Org. Lett., 2013, 15, 3844 CrossRef CAS PubMed.
- R. Ziessel, G. Ulrich, A. Haefele and A. Harriman, J. Am. Chem. Soc., 2013, 135, 11330 CrossRef CAS PubMed.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77 RSC.
- B. S. Kim, B. W. Ma, V. R. Donuru, H. Y. Liu and J. M. J. Frechet, Chem. Commun., 2010, 46, 4148 RSC.
- T. Bura, N. Leclerc, S. Fall, P. Leveque, T. Heiser, P. Retailleau, S. Rihn, A. Mirloup and R. Ziessel, J. Am. Chem. Soc., 2012, 134, 17404 CrossRef CAS PubMed.
- A. Bessette and G. S. Hanan, Chem. Soc. Rev., 2014, 43, 3342 RSC.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed.
- V. R. Donuru, G. K. Vegnesa, S. Velayudham, S. Green and H. Liu, Chem. Mater., 2009, 21, 2130 CrossRef CAS.
- G. Meng, S. Velayudham, A. Smith, R. Luck and H. Liu, Macromolecules, 2009, 42, 1995 CrossRef CAS.
- Y. Wang, J. Chen, Y. Zhen, H. Jiang, G. Yu, Y. Liu, E. Baranoff, H. Tan and W. Zhu, Mater. Lett., 2015, 139, 130 CrossRef CAS PubMed.
- F. Cheng and F. Jakle, Polym. Chem., 2011, 2, 2122 RSC.
- T. Rousseau, A. Cravino, E. Ripaud, P. Leriche, S. Rihn, A. de Nicola, R. Ziessel and J. Roncali, Chem. Commun., 2010, 46, 5082 RSC.
- C. J. Wood, G. H. Summers and E. A. Gibson, Chem. Commun., 2015, 51, 3915 RSC.
- Y. Ooyama, Y. Hagiwara, T. Mizumo, Y. Harima and J. Ohshita, New J. Chem., 2013, 37, 2479 RSC.
- H. B. Zhao, J. X. Liao, J. H. Ning, Y. J. Xie, Y. J. Cao, L. Chen, D. L. Yang and B. Y. Wang, Adv. Synth. Catal., 2010, 352, 3083 CrossRef CAS PubMed.
- A. Burghart, H. J. Kim, M. B. Welch, L. H. Thorensen, J. Reibenspies, K. Burgess, F. Bergstrom and L. B. A. Johansson, J. Org. Chem., 1999, 64, 7813 CrossRef CAS.
- T. Karstens and K. Kobs, J. Phys. Chem., 1980, 84, 1871 CrossRef CAS.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899 CrossRef PubMed.
- B. C. Popere, A. M. D. Pelle, A. Poe, G. Balaji and S. Thayuamanavan, Chem. Sci., 2012, 3, 3093 RSC.
- A. Mirloup, N. Leclerc, S. Rihn, T. Bura, R. Bechara, A. Hebraud, P. Leveque, T. Heiser and R. Ziessel, New J. Chem., 2014, 38, 3644 RSC.
- J. E. Coughlin, Z. B. Henson, G. C. Welch and G. C. Bazan, Acc. Chem. Res., 2013, 47, 257 CrossRef PubMed.
- Y. Chen, X. Wan and G. Long, Acc. Chem. Res., 2013, 46, 2645 CrossRef CAS PubMed.
- Y. S. Liu, C.-C. Chen, Z. R. Hong, J. Gao, Y. Yang, H. P. Zhou, L. T. Dou and G. Li, Sci. Rep., 2013, 3, 3356 Search PubMed.
- Q. Zhang, B. Kan, F. Liu, G. Long, X. Wan, X. Chen, Y. Zuo, W. Ni, H. Zhang, M. Li, Z. Hu, F. Huang, Y. Cao, Z. Liang, M. Zhang, T. P. Russell and Y. Chen, Nat. Photonics, 2014, 9, 35 CrossRef PubMed.
- B. Kan, Q. Zhang, M. Li, X. Wan, W. Ni, G. Long, Y. Wang, X. Yang, H. Feng and Y. Chen, J. Am. Chem. Soc., 2014, 136, 15529 CrossRef CAS PubMed.
- J. Zhou, Y. Zuo, X. Wan, G. Long, Q. Zhang, W. Ni, Y. Liu, Z. Li, G. He, C. Li, B. Kan, M. Li and Y. Chen, J. Am. Chem. Soc., 2013, 135, 8484 CrossRef CAS PubMed.
- L. Chen, L. Huang, D. Yang, S. Ma, X. Zhou, J. Zhang, G. Tu and C. Li, J. Mater. Chem. A, 2014, 2, 2657 CAS.
- Y. Lin, P. Cheng, Y. Li and X. Zhan, Chem. Commun., 2012, 48, 4773 RSC.
- H. Hoppe and N. S. Sariciftci, J. Mater. Res., 2004, 19, 1942 CrossRef.
- X.-C. Li, Y. Liu, M. S. Liu and A. K.-Y. Jen, Chem. Mater., 1999, 11, 1568 CrossRef CAS.
- W. J. Liu, M. Pink and D. Lee, J. Am. Chem. Soc., 2009, 131, 8703 CrossRef CAS PubMed.
- J. Liao, Y. Wang, Y. Xu, H. Zhao, X. Xiao and X. Yang, Tetrahedron, 2015, 71, 5078 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15389a |
| This journal is © The Royal Society of Chemistry 2015 |