Morphology and size control of calcium carbonate crystallized in a reverse micelle system with switchable surfactants
Jianzhong Jiang*a,
Yuxuan Maa,
Ting Zhanga,
Zhengyong Liang*b and
Zhenggang Cuia
aThe Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, 1800 Lihu Road, Wuxi, Jiangsu, P. R. China. E-mail: j.z.jiang@hotmail.com
bSchool of Chemical Engineering and Energy, Zhengzhou University, No 100 Science Road, Zhengzhou, Henan, P. R. China. E-mail: 31370056@qq.com
First published on 9th September 2015
Abstract
A series of CaCO3 particles with different morphologies, such as rhombohedron, sphere, and dendrite-like, were successfully prepared in CO2/N2 switchable surfactant (N′-dodecyl-N,N-dimethyl acetamidine bicarbonate) reverse micelles.
Biologically mineralized materials have attracted considerable attention in recent years because of their unusual properties arising from their complex shape, hierarchical organization, and various polymorphs of their constituent minerals.1–5 As one of the most common biological minerals, calcium carbonate (CaCO3) has attracted considerable attention due to its wide application in industrial and scientific fields. CaCO3 crystals exist in three main crystalline polymorphs (calcite, vaterite, and aragonite) and in amorphous material as well. Calcite and aragonite are the main forms existing in organisms, and from a thermodynamics perspective, calcite is more stable than aragonite. The properties and applications of CaCO3 depend on the morphology, polymorph, particle size, and chemical purity of the crystals.6–10
Currently, water-in-oil (W/O) microemulsions have been given considerable attention for their soft template effect, reproducibility, and simple maneuverability.11 W/O microemulsions are composed of nanometer-sized water droplets that are dispersed in a continuous oil medium and stabilized by surfactant molecules. Such thermodynamically stable systems are heterogeneous on a molecular scale and able to serve as nanoreactors, which favor the growth of small crystallites with a desired narrow size distribution and morphology.12,13 However, this method requires a certain amount of an oil phase and surfactant. How to efficiently separate, recover, and simply remove the surfactant is an important issue to be solved.
As a switchable surfactant can undergo reversible inter-conversions between active and inactive forms,14 it is possible to recover and re-use the surfactant afterwards and to easily remove it from the system. Jessop et al. reported a switchable surfactant using CO2/N2 as a trigger,15–17 where the hydrophobic long-chain alkyl amidines (inactive) could be protonated and become water-soluble surfactants upon the addition of CO2, and the amidinium ions (active) could then be deprotonated by bubbling N2. Both CO2 and N2 are inexpensive and environmentally benign.
Herein, we attempt to find a relatively simple, mild, low-cost, and environmentally friendly strategy to synthesize CaCO3 nanoparticles. A series of CaCO3 particles were successfully synthesized in CO2/N2 switchable surfactant (N′-dodecyl-N,N-dimethyl acetamidine bicarbonate) reverse micelles. The switchable surfactant can form reverse micelles in the oil phase spontaneously. At the completion of the reaction, N2 is introduced to switch off the “active button”. Particles can be obtained by simply washing to remove the adsorbed surfactants, which simplifies the tedious traditional separation process.18,19
Reverse micellar solutions were prepared by adding an aqueous solution of either CaCl2 or Na2CO3 to a certain concentration of N′-dodecyl-N,N-dimethylethyl amidine bicarbonate/n-heptane/n-hexanol mixtures. Then, the two micellar solutions, containing CaCl2 and Na2CO3, were mixed and stirred for 6 h at room temperature (Scheme 1). After completion of the reaction, the mixture was heated to 65 °C and N2 was bubbled for 30 min until the system became turbid. Calcium carbonate was separated with centrifugation (8000 rpm min−1). The separated calcium carbonate particles were washed with ethanol and acetone alternately 2–3 times and dried before characterization.
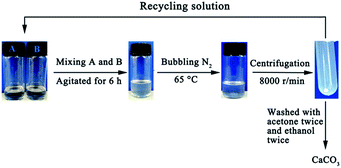 | ||
| Scheme 1 Synthesis of CaCO3 in CO2/N2 switchable surfactant reverse micelles (“A” refers to the reverse micelle of CaCl2; “B” refers to the reverse micelle of Na2CO3). | ||
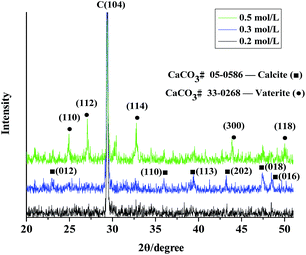
The X-ray diffraction (XRD) patterns of CaCO3 synthesized in the reverse micelles at different reactant concentrations are shown in Fig. 1. The results demonstrated that pure calcite was produced when the reactant concentration was 0.2 M and 0.3 M. With the increase in reactant concentration, the relative intensity of each peak was enhanced. When the concentration reached 0.5 M, a mixture composed of calcite and vaterite was fabricated (Table 1).
| Reactant concentrations (mol L−1) | Calcite (%) | Vaterite (%) |
|---|---|---|
| a The surfactant concentration is 0.07 M; the concentration of CaCl2 and Na2CO3 is same; molar ratio [CaCl2]/[Na2CO3] = 1. | ||
| 0.2 | 100 | 0 |
| 0.3 | 100 | 0 |
| 0.5 | 67.8 | 32.2 |
Although calcite is more stable than other polymorphs at ambient temperature and atmospheric pressure, precipitates may be found as a mixture of polymorphs or with one of the forms predominating.20 Ostwald proposed that the solid first formed on crystallization of a melt or a solution would be the least stable polymorph.21 Therefore, in this experiment, the first phase precipitated out of the supersaturated solution was amorphous calcium carbonate and then transformed into vaterite as a metastable (more soluble) phase. The transformation of vaterite into calcite may suggest that the more soluble vaterite crystals dissolve, whereas the less soluble calcite crystals nucleate and grow.22 When the concentration of reactant was high, vaterite is precipitated in appreciable amounts, confirming that vaterite formation favors high (relative) concentrations of Ca2+.23
As shown in Fig. 2, the reactant concentration also had a strong influence on the shape of the crystals. When the reactant concentration was 0.5 M, CaCO3 was spherical (typical vaterite morphology) (Fig. 2a), accompanied by a few rhombohedral crystals (typical calcite morphology),24 which correspond to that seen in the XRD patterns. Under 0.3 M, the obtained CaCO3 crystals were almost rhombohedra, with smaller ones gathered together forming larger ones. This was mainly due to the high surface energy of the fine calcium carbonate crystals, tending to aggregate into larger particles to reduce the surface energy. When the concentration was decreased to 0.2 M, rod-like particles appeared. It has to be pointed out that the exact growth mechanism is still unknown, although some explanations have been given in the literature based on two types of rates, which denote the rate of nucleation and growth outward at the ends of rod-like primary crystals (r1) and the rate of nucleation and growth along the rod axes of rod-like primary crystals (r2), respectively. When r1 is much smaller than r2, rod-like or ellipsoid crystals will be obtained.25,26
 | ||
| Fig. 2 SEM images of CaCO3 prepared at different reactant concentrations. (a) 0.5 mol L−1, (b) 0.3 mol L−1, (c) 0.2 mol L−1. | ||
Fig. 3 shows the XRD patterns of CaCO3 prepared at different surfactant concentrations. It indicates that when the surfactant concentration was 0.04 M, the calcite and vaterite crystalline mixture was obtained. With the increase of surfactant concentration, the mixed polymorph turns into pure calcite (Table 2). This may be due to electrostatic interaction. The cationic surfactant is easily adsorbed on the negatively charged surface; therefore, the crystal tends to be the most stable crystalline form of calcite, and any excess surfactant will adsorb on the surface of calcium carbonate, resulting in polymorph changes.
| Surfactant concentration (mol L−1) | Calcite (%) | Vaterite (%) |
|---|---|---|
| a The reactant concentration is 0.3 M; molar ratio [CaCl2]/[Na2CO3] = 1. | ||
| 0.04 | 78.5 | 21.5 |
| 0.07 | 100 | 0 |
| 0.1 | 100 | 0 |
The aggregation number of micelles varies with the different surfactant concentrations, thus the constitute of the “pool” size and shape are different. When the surfactant concentration is 0.1 M, the dendritic structure composed of needle-like aggregates can be observed, as in Fig. 4a. From the magnified image, the diameters of the branches are to be under 200 nm. Aggregates with the same morphology are also obtained by β-CD and DTAB.27 In the present research, a unique morphology was obtained in an appropriate concentration without any complexes or additives. When the surfactant concentration is higher, the degree of supersaturation of Ca2+ will increase, especially on the side of needles. This tends to cause the two-dimensional nucleation, forming a dendritic morphology. The mixture of rhombohedra and hollow pie-like particles exist at 0.04 M. Similar hollow structures of vaterite crystal types have also been reported.28
 | ||
| Fig. 4 SEM images of CaCO3 prepared at different surfactant concentrations (a) 0.1 M, (b) magnification of 0.1 M, (c) 0.07 M, (d) 0.04 M. | ||
Surfactants, with hydrophilic and hydrophobic parts, dissolved in organic solvents form spheroidal aggregates called reverse micelles. Water is readily solubilized in the polar core, forming a so-called water pool, characterized by ω, which is the molar ratio of water to surfactant.29 The size of the “water pool” increases with ω. Since the calcium carbonate particles are generated in the “pool”, the size of the pool directly determines the size of the calcium carbonate particles. Fig. 5 shows the XRD patterns of CaCO3 obtained with different ω. When ω reaches 3.52, the CaCO3 particles show a mixed crystal type of vaterite and calcite. From the corresponding SEM images in Fig. 6(b), a larger size of rhombohedra appear, which is not due to the smaller ones uniting together forming a larger one. In addition, the change of ω also affects the strength of the reverse micelle interfacial membrane. In general, as ω increases, the interfacial membrane strength becomes smaller.30 As a result, the interface membrane is easily broken because of the collision between the reverse micelles, and the particle size becomes large and difficult to control.
 | ||
| Fig. 5 XRD patterns of CaCO3 prepared at different ω. The reactant concentration is 0.3 mol L−1 and molar ratio [CaCl2]/[Na2CO3] = 1; the surfactant concentration is 0.07 M. | ||
Conclusions
In conclusion, switchable surfactant N′-dodecyl-N,N-dimethylethyl amidine bicarbonate reverse micelles were used as soft templates to prepare submicron fine calcium carbonate. Particles with rhombohedron, sphere, and dendrite-like morphologies were obtained by changing the reactant concentration and surfactant concentration, ω. Compared with other methods, the reaction conditions are relatively mild. This research provides new insights into a facile preparation of calcium carbonate particles and might well be extendable to the morpho-synthesis of other novel inorganic materials.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 20901032 and 21473080), the Fundamental Research Funds for the Central Universities (No. JUSRP51405A) and the MOE & SAFEA for the 111 Project (B13025) is gratefully acknowledged.Notes and references
- E. Dalas, P. Klepetsanis and P. G. Koutsoukos, Langmuir, 1999, 15, 8322 CrossRef CAS.
- M. Kitamura, J. Colloid Interface Sci., 2001, 236, 318 CrossRef CAS PubMed.
- Y. S. Han, G. Hadiko and M. Fuji, J. Eur. Ceram. Soc., 2006, 26, 843 CrossRef CAS PubMed.
- H. Cölfen and L. Qi, Chem.–Eur. J., 2001, 7, 106 CrossRef.
- D. Liu and M. Z. Yates, Langmuir, 2006, 22, 5566 CrossRef CAS PubMed.
- L. Liu, D. Fan and H. Mao, J. Colloid Interface Sci., 2007, 306, 154 CrossRef CAS PubMed.
- J. Yu, M. Lei and B. Cheng, J. Cryst. Growth, 2004, 261, 566 CrossRef CAS PubMed.
- O. Grassmann and P. L. Obmann, Biomaterials, 2004, 25, 277 CrossRef CAS.
- J. W. Ahn and K. S. Choi, J. Am. Ceram. Soc., 2004, 87, 286 CrossRef CAS PubMed.
- Q. Li, Y. Ding and F. Li, J. Cryst. Growth, 2002, 236, 357 CrossRef CAS.
- M. P. Pileni, Nat. Mater., 2003, 2, 145 CrossRef CAS PubMed.
- M. P. Pileni, Langmuir, 2001, 17, 7476 CrossRef CAS.
- M. L. Rock, L. J. Tranchitella and R. S. Pilato, Colloid Polym. Sci., 1997, 275, 893 CAS.
- P. Brown, C. P. Butts and J. Eastoe, Soft Matter, 2013, 9, 2365 RSC.
- Y. X. Liu, P. G. Jessop, M. Cunningham, C. A. Eckert and C. L. Liotta, Science, 2006, 313, 958 CrossRef CAS PubMed.
- C. Liang, J. R. Harjani, T. Robert, E. Rogel, D. Kuehne, C. Ovalles, V. Sampath and P. G. Jessop, Energy Fuels, 2012, 26, 488 CrossRef CAS.
- M. Mihara, P. Jessop and M. Cunningham, Macromolecule, 2011, 44, 3688 CrossRef CAS.
- J. Z. Jiang, Y. E. He, L. P. Wan, Z. Cui, Z. G. Cui and P. G. Jessop, Chem. Commun., 2013, 49, 1912 RSC.
- J. Jiang, Y. Zhu and Z. Cui, Angew. Chem., Int. Ed., 2013, 52, 12373 CrossRef CAS PubMed.
- C. Y. Tai and F. B. Chen, AIChE J., 1998, 44, 1790 CrossRef CAS PubMed.
- T. Threlfall, Org. Process Res. Dev., 2003, 7, 1017 CrossRef CAS.
- N. Spanos and P. G. Koutsoukos, J. Cryst. Growth, 1998, 191, 783 CrossRef CAS.
- N. Spanos and P. G. Koutsoukos, Am. Mineral., 1974, 9, 947 Search PubMed.
- P. Liang, Y. Zhao and Q. Shen, J. Cryst. Growth, 2004, 261, 71–576 Search PubMed.
- J. Yu, X. Zhao, B. Cheng and Q. Zhang, J. Solid State Chem., 2005, 178, 861 CrossRef CAS PubMed.
- Z. Xue, B. Hu, S. Dai and Z. Du, Mater. Sci. Eng. C, 2015, 55, 506 CrossRef CAS PubMed.
- H. Jia, X. Bai and L. Zheng, CrystEngComm, 2011, 13, 7252 RSC.
- Y. Yao, W. Dong and S. Zhu, Langmuir, 2009, 25, 13238 CrossRef CAS PubMed.
- M. P. Pileni, I. Lisiecki, L. Motte, C. Petit, J. Cizeron, N. Moumen and P. Lixon, Prog. Colloid Polym. Sci., 1993, 93, 1 CAS.
- H. C. Zhou, J. Zhuang, X. Wang, J. Xu and Y. D. Li, Acta Chim. Sin., 2003, 61, 372 CAS.
| This journal is © The Royal Society of Chemistry 2015 |