Carbon nanotube-based QuEChERS extraction and enhanced product ion scan-assisted confirmation of multi-pesticide residue in dried tangerine peel†
Xiaowen Doua,
Xianfeng Chua,
Weijun Konga,
Yinhui Yanga and
Meihua Yang*ab
aInstitute of Medicinal Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100193, China. E-mail: yangmeihua15@hotmail.com; Fax: +86 10 62896288; Tel: +86 10 57833277
bHainan Branch Institute of Medicinal Plant Development, Chinese Academy of Medicinal Sciences & Peking Union Medical College, Wanning 571533, Hainan, China
First published on 6th October 2015
Abstract
A sorbent package consisting of a combination of multiwalled carbon nanotubes (MWNTs) and a primary secondary amine (PSA) has been used for a modified quick, easy, effective, rugged, and safe (QuEChERS) extraction of 104 pesticides from dried tangerine peel samples. MWNTs and graphitized carbon black (GCB) have been compared in terms of purification efficiency and recovery; the best results were achieved with MWNTs. The pesticides were quantified on a liquid chromatography tandem mass spectrometry (LC-MS/MS) system in scheduled multiple reaction monitoring mode, and identified on the basis of product ion abundance ratios as well as characteristic fragments in enhanced product ion spectra. Calibration curves for most of the analytes showed correlation coefficients better than 0.9916. Detection limits ranged from 0.2 to 40 μg kg−1. Good precision was achieved with relative standard deviations of less than 20%. Results of accuracy in spiked samples were in the range 71.1–117.6%, except for pesticides such as thiabendazole, teflubenzuron, hexaflumuron, and methomyl. The proposed method has been applied to 57 dried tangerine peel samples from the Chinese market; 16 pesticides were detected, including carbendazim, chlorpyrifos, isoprothiolane and methidathion, at levels that exceeded the recommended maximum residue limits in some samples. The newly established method has advantages of good recovery and a rapid clean-up procedure, showing enhanced product ion scan-assisted confirmation to be a useful tool for obtaining reliable results.
1 Introduction
Dried tangerine peel is popular as a dietary supplement in Asian countries, such as Korea, China, and Japan,1 and is used as an ingredient in spices, condiments, snack food, and tea in many countries. To ensure yields and quality, pesticides have become the most common measure to control insects and diseases. However, deviations from good agricultural practice in the use of pesticides readily leads to pesticide residues, which may pose a potential threat to human health. To ensure that these residues are kept below tolerated levels, the European Commission has stipulated maximum residue levels (MRLs) for some pesticides as low as 0.01 mg kg−1 in the regulation (EC) no. 396/2005,2,3 yet only a few methods are available for evaluation of the broadly contaminated multiclass pesticides in dried tangerine peel.4 In this context, the development of reliable and accurate methods is necessary for monitoring the levels of pesticide residues.Sample preparation plays a key role in separating trace pesticides from matrices and maintenance of chromatographic systems. Many preparation techniques, such as solid-phase extraction (SPE),5,6 gel-permeation chromatography (GPC),7 matrix solid-phase dispersion extraction (MSPD),8 supercritical fluid extraction (SFE),9 solid-phase microextraction (SPME),10 and liquid–liquid extraction (LLE),11 have been reported for the determination of pesticides. Some of the aforementioned methods involve large amounts of organic solvents (LLE), special equipment (GPC and SFE), are labor-intensive (MSPD), or incur high costs (SPE), which has limited their use in relation to complicated matrices. The quick, easy, cheap, effective, rugged, and safe (QuEChERS) method, a combination of extraction and purification, has been widely accepted by the international community by virtue of providing high recovery, super efficiency, and good reproducibility. In the QuEChERS procedure, a primary secondary amine (PSA) is most commonly used as a polar adsorbent to remove organic acids, fatty acids, sugars, and polar pigments.12,13 Depending on the nature of the chemicals and matrices, graphitized carbon black (GCB)14,15 has also been proposed to eliminate non-polar co-extracts, especially plant pigments. To achieve good performance in the preparation step, the pursuit of novel sorbents is ongoing. Multi-walled carbon nanotubes (MWNTs), as novel carbon nanomaterials, consist of multiple layers of carbon atoms rolled up into nanoscale tubes.16 Owing to their extremely large surface area, electron-rich nature, and hydrophobicity, MWNTs possess excellent adsorptive capabilities and can serve as a perfect extraction material. MWNTs as sorbents have been employed in SPE,17–20 MSPD,21,22 and dispersive solid-phase extraction (dSPE).23 In most such previous studies, MWNTs have been used alone as a sorbent in a QuEChERS step.24,25 Only a few papers have dealt with evaluation of a combination of MWNTs with other sorbents in matrix effect reduction.26 To the best of our knowledge, the removal of interfering species by MWNTs and GCB has not hitherto been compared. In addition, there has been no report on the use of MWNTs for pesticide determination in dried tangerine peel by the QuEChERS method.
For the quantification of multi-pesticide residues, especially by high-throughput analytical approaches, the primary focus has been on gas chromatography-mass spectrometry (GC-MS) and liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS).27 LC-MS/MS is preferred over GC-MS in terms of a wider scope of targets, increased sensitivity, and better selectivity.27 Among available LC-MS/MS techniques for the identification and quantification of unknown chemicals, the hybrid-quadrupole linear ion trap tandem MS (QqLIT-MS/MS) system has recently been suggested for application in drug discovery, the screening of active compounds, and the detection of contaminants.28–32 This system allows scheduled multiple reaction monitoring simultaneously with information-dependent acquisition-triggered enhanced product ion scan (scheduled MRM-IDA-EPI). In scan mode, quantitative data from MRM chromatography and data on fragments identified from the EPI mass spectrum can be acquired within the same cycle.
In the present study, a sorbent package consisting of MWNTs and PSA has been examined in combination with the QuEChERS method for the extraction and purification of 104 pesticides. QqLIT-MS/MS has been used to quantify the levels of pesticide residues in scheduled MRM mode. The pesticides have been identified on the basis of product ion abundance ratios as well as characteristic fragments from EPI spectra.
2 Experimental
2.1 Standards
All pesticide standards were provided by the Agro-Environmental Protection Institute (Tianjin, China). On the basis of their solubility, individual stock standard solutions of pesticides were prepared in methanol, acetone, or n-hexane, each at a concentration of 100 μg mL−1. Intermediate solutions of each pesticide at a concentration of 50 ng mL−1 were prepared in a mixture of acetonitrile/water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) to optimize the MS/MS conditions. Prior to simultaneous analysis, a stock multi-standard solution containing 500 ng mL−1 of each pesticide was prepared in methanol, stored at −20 °C, and used within 1 month. To avoid degradation of pesticides, standard working solutions at various concentrations were prepared daily by appropriate dilution of the stock multi-standard solution in blank matrix extract or a mixed solvent of acetonitrile/water (3
50, v/v) to optimize the MS/MS conditions. Prior to simultaneous analysis, a stock multi-standard solution containing 500 ng mL−1 of each pesticide was prepared in methanol, stored at −20 °C, and used within 1 month. To avoid degradation of pesticides, standard working solutions at various concentrations were prepared daily by appropriate dilution of the stock multi-standard solution in blank matrix extract or a mixed solvent of acetonitrile/water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v).
2, v/v).
2.2 Reagents and chemicals
Chromatography grade acetonitrile and methanol were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Cleanert PSA (40–60 μm) and Cleanert GCB (200–400 mesh, specific surface area 100 m2 g−1) were purchased from Bonna-Agela Technologies (Tianjin, China). Multi-walled carbon nanotubes (outer diameter 10–20 nm, length 10–30 μm, specific surface area > 200 m2 g−1) were procured from Beijing Dk Nano technology Co., Ltd (Beijing, China). Other reagents and chemicals were of analytical grade. Formic acid was supplied by Xilong Chemical (Guangdong, China). Anhydrous magnesium sulfate (anh. MgSO4) and sodium chloride (NaCl) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).2.3 Apparatus and analytical conditions
The ultrafast liquid chromatography (UFLC) system consisted of an LC-20A pump, an SIL-20AC autosampler, a DGU-20 A3 degasser, and a CTO-20A column oven (Shimadzu, Japan). Multiple pesticides were separated on an AQUITY UPLC BEH Shield RP18 column (100 × 2.1 mm, 1.7 μm) from Waters (Massachusetts, USA) by using a binary mobile phase composed of 0.1% (v/v) formic acid in acetonitrile (A) and 0.1% (v/v) formic acid in water (B) with the following gradient elution program: 0 min, 90% B; 3.5 min, 55% B; 5 min, 50% B; 15 min, 25% B; 16 min 1% B; 18 min 1% B; 19 min 90% B; 22 min 90% B, at a flow rate of 0.3 mL min−1. The injection volume was 10 μL.An Applied Biosystems Sciex QTRAP® 5500 MS/MS spectrometer equipped with a version of 1.6 Analyst software (AB SCIEX, Massachusetts, USA) was employed in the analysis. Pesticides were protonated by an electrospray ionization (ESI) source in positive mode. High purity (99.999%) nitrogen was used as curtain gas (CUR) and collision gas (CAD), while compressed air was used as nebulizer gas (GS1) and heating gas (GS2). The ionization source-dependent parameters were set as follows: ion spray voltage, 5500 V; source temperature (TEM) 550 °C; CUR, 35 psi; CAD, medium; GS1 and GS2, each 50 psi. Scheduled MRM mode was selected, with 120 s as the MRM detection window and 0.8 s as the target scan time.
Fragment-rich EPI spectra were collected through information-dependent acquisition (IDA) experiments, whereby a full scan of the precursor ion for each pesticide was triggered in conjunction with scheduled MRM mode. The IDA criteria included selecting the two most intense precursor ions after dynamic background subtraction of the survey scan, and never excluded the former target ions. Mass tolerance for precursor ions was 250 mDa. EPI spectra were acquired by intensity exceeding 500 counts per second (cps) over a mass range of m/z 50–600 for product ions at a scan rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da s−1. The collision energy (CE) and CAD were set at 35 V and high, respectively.
000 Da s−1. The collision energy (CE) and CAD were set at 35 V and high, respectively.
2.4 Sample preparation
A total of 57 batches of dried tangerine peel samples were collected from markets and drugstores across China. Samples were homogenized, ground, and passed through a 65-mesh sieve before analysis. An accurately weighed powder sample (1.0 g) was immersed in 2.5 mL of purified water for 1 min and then extracted by vigorously shaking for 1 min after the addition of acetonitrile (10 mL). NaCl (0.5 g) and anh. MgSO4 (2.0 g) were added to the mixture with continuous shaking for 1 min to facilitate transfer of the targets into acetonitrile and removal of water from the acetonitrile. The mixture was then centrifuged for 5 min at 4000 rpm. An aliquot of extract (1 mL) was cleaned up by a mixture of 25 mg PSA, 10 mg MWNTs, and 150 mg anh. MgSO4, and the supernatant was concentrated to dryness under a nitrogen flow at 40 °C. The residue was redissolved in 1 mL of acetonitrile/water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) and the solution was filtered through a 0.22 μm membrane for later use. To investigate the potential benefits of using MWNTs as an alternative absorbent to GCB in the QuEChERS method, the fortified sample was extracted and purified according to the above procedure but using GCB in place of MWNTs.
2, v/v) and the solution was filtered through a 0.22 μm membrane for later use. To investigate the potential benefits of using MWNTs as an alternative absorbent to GCB in the QuEChERS method, the fortified sample was extracted and purified according to the above procedure but using GCB in place of MWNTs.
3 Results and discussion
3.1 Optimization of MS/MS conditions
The pesticides investigated in dried tangerine peel in this study were selected according to registered pesticides on the China pesticide information network, as well as compounds required to be regulated, specially monitored, prohibited, or limited according to the U.S. Environmental Protection Agency (U.S. EPA), European Union (EU), and China authority. To ensure production output and the quality, almost 90 kinds of pesticides were registered for tangerines in Ministry of Agriculture of the People's Republic of China. As one of the main tangerine cultivation countries, China has widely export the fruits to surrounding nations such as Southeast Asia and Russia. Considering the risk of co-occurrence of those multi-pesticides, a total of 132 pesticides composed of a variety of structural classes, such as organophosphorus, carbamates, triazoles, benzinidazoles, anilines, pyridines, and strobilurins, are in current use or intensively monitored in raw materials from tangerines. MS conditions play an important role in the sensitivity of trace substance detection. In this study, the MS/MS parameters were optimized to efficiently produce the characteristic fragment ions by tuning with each standard using a syringe pump. As a result, 28 pesticides were excluded from the analysis because no precursor was found (cartap, chlorothalonil, cypermethrin, etc.), there were no fragments or only one for analysis (formothion, parathion-methyl, benomyl, etc.), no peak was detected (propamocarb), or the intensity was too low (bifenthrin) under LC-MS/MS conditions. For accurate quantification and confirmation of the remaining analytes, two ion transitions per pesticide were monitored, and the optimal MS/MS parameters included declustering potential (DP), collision energy (CE), and collision cell exit potential (CXP), together with scan time, as listed in Table 1.| Pesticides | Structure category | Molecular formula | Retention/min | Q transition | CE1/V | q transition | CE2/V | DP/V | CXP/V |
|---|---|---|---|---|---|---|---|---|---|
| a Note: BEZs, benzinidazoles; BPUs, benzoylureas; CAMs, carbamates; OPPs, organophosphorus; PYHs, pyrethroids; Q, quantification ion; q, qualification ion; DP, declustering potential, CE, collision energy and CXP, cell exit potential. | |||||||||
| Carbendazim | BEZs | C9H9N3O2 | 5.0 | 192/160 | 22 | 192/132 | 41 | 105 | 9 |
| Thiabendazole | BEZs | C10H7N3S | 5.2 | 202/175.1 | 34 | 202/131 | 43 | 130 | 9 |
| Thiophanate-methyl | BEZs | C12H14N4O4S2 | 7.7 | 343/151 | 24 | 343/311 | 14 | 117 | 12 |
| Chlorfluazuron | BPUs | C20H9Cl3F5N3O3 | 17.6 | 540/383 | 24 | 540/158.2 | 23 | 84 | 10 |
| Flufenoxuron | BPUs | C21H11ClF6N2O3 | 16.7 | 489.2/158.1 | 22 | 489.2/141.1 | 33 | 91 | 13 |
| Hexaflumuron | BPUs | C16H8Cl2F6N2O3 | 17.4 | 461/141.1 | 30 | 461/158.1 | 22 | 90 | 12 |
| Teflubenzuron | BPUs | C14H6Cl2F4N2O2 | 14.5 | 381.2/158.1 | 23 | 381.2/141.1 | 30 | 84 | 13 |
| Triflumuron | BPUs | C15H10ClF3N2O3 | 13.1 | 359.3/156.2 | 22 | 359.3/138.8 | 22 | 103 | 10 |
| Aldicarb | CAMs | C7H14N2O2S | 7.1 | 213.1/88.9 | 21 | 213.1/116 | 15 | 124 | 10 |
| Aldoxycarb | CAMs | C7H14N2O4S | 5.5 | 223.1/86 | 17 | 223.1/88.9 | 12 | 63 | 9 |
| Bendiocarb | CAMs | C11H13NO4 | 8.0 | 224.1/167.1 | 11 | 224.1/108.9 | 21 | 87 | 8 |
| Carbaryl | CAMs | C12H11NO2 | 8.5 | 202.1/145 | 17 | 202.1/127.1 | 42 | 74 | 12 |
| Carbofuran | CAMs | C12H15NO3 | 7.9 | 222.1/165 | 16 | 222.1/122.9 | 28 | 71 | 8 |
| Carbosulfan | CAMs | C20H32N2O3S | 18.6 | 381.2/118 | 23 | 381.2/160 | 19 | 98 | 12 |
| Fenobucarb | CAMs | C12H17NO2 | 9.7 | 208.1/94.9 | 18 | 208.1/152 | 9 | 118 | 12 |
| Furathiocarb | CAMs | C18H26N2O5S | 14.6 | 383.1/195 | 26 | 383.1/252 | 17 | 130 | 11 |
| Indoxacarb | CAMs | C22H17ClF3N3O7 | 14.2 | 528/149.8 | 31 | 528/217.9 | 30 | 100 | 10 |
| Isoprocarb | CAMs | C11H15NO2 | 8.8 | 194.1/95 | 19 | 194.1/137.1 | 13 | 95 | |
| Methiocarb | CAMs | C11H15NO2S | 9.9 | 226/121.1 | 23 | 226/169.2 | 12 | 101 | 12 |
| Methomyl | CAMs | C5H10N2O3S | 5.6 | 163/87.9 | 13 | 163/106 | 14 | 100 | 12 |
| Metolcarb | CAMs | C9H11NO2 | 7.6 | 166/108.8 | 13 | 166/90.9 | 32 | 117 | 13 |
| Oxamyl | CAMs | C7H13N3O3S | 5.4 | 220/72 | 20 | 220/90 | 12 | 83 | 12 |
| Pirimicarb | CAMs | C11H18N4O2 | 5.4 | 239.1/71.9 | 25 | 239.1/182.1 | 20 | 81 | 8 |
| Propoxur | CAMs | C11H15NO3 | 7.8 | 210.1/111.1 | 17 | 210.1/168.1 | 11 | 67 | 12 |
| Thiodicarb | CAMs | C10H18N4O4S3 | 7.6 | 355/87.9 | 25 | 355/107.9 | 19 | 104 | 12 |
| Diethofencarb | CAMs | C14H21NO4 | 9.9 | 268.1/226 | 13 | 268.1/180.1 | 23 | 90 | 12 |
| Acephate | OPPs | C4H10N3PS | 1.4 | 184/143.1 | 11 | 184/125 | 23 | 78 | 10 |
| Azinphos ethyl | OPPs | C12H16N3O3PS2 | 11.8 | 346.1/131.9 | 21 | 346.1/260.9 | 11 | 51 | 12 |
| Azinphos-methyl | OPPs | C10H12N3O3PS2 | 10.1 | 318.2/132 | 20 | 318.2/77 | 45 | 74 | 12 |
| Chlorfenvinfos | OPPs | C12H14Cl3O4P | 11.6 | 359/155 | 17 | 359/127.1 | 24 | 91 | 13 |
| Clorpyrifos | OPPs | C9H11Cl3NO3PS | 15.8 | 350.1/197.9 | 24 | 350.1/96.9 | 40 | 72 | 9 |
| Clorpyrifos-methyl | OPPs | C7H7Cl3NO3PS | 13.7 | 322.2/125.1 | 26 | 322.2/289.8 | 20 | 70 | 13 |
| Coumaphos | OPPs | C14H16ClO5PS | 13.4 | 363.2/226.9 | 33 | 363.2/306.9 | 23 | 130 | 12 |
| Demeton | OPPs | C16H38O6P2S4 | 8.7 | 259.1/89 | 18 | 259.1/60.9 | 43 | 53 | 10 |
| Diazinon | OPPs | C12H21N2O3PS | 12.2 | 305.1/168.9 | 27 | 305.1/153.2 | 27 | 110 | 10 |
| Dichlofenthion | OPPs | C10H13Cl2O3PS | 15.7 | 315.1/258.8 | 20 | 315.1/287 | 14 | 56 | 13 |
| Dichlorvos | OPPs | C4H7Cl2O4P | 7.5 | 220.9/109 | 20 | 220.9/145 | 16 | 120 | 10 |
| Dicrotophos | OPPs | C8H16NO5P | 5.6 | 238/112 | 16 | 238/192.9 | 13 | 70 | 11 |
| Dimethoate | OPPs | C5H12NO3PS2 | 6.6 | 230/199 | 12 | 230/171 | 18 | 70 | 10 |
| Disulfoton | OPPs | C8H19O2PS3 | 13.7 | 275.1/89 | 21 | 275.1/61.2 | 21 | 54 | 12 |
| Ethion | OPPs | C9H22O4P2S4 | 16.1 | 385.1/198.9 | 12 | 385.1/1171.1 | 21 | 90 | 10 |
| Ethoprophos | OPPs | C8H19O2PS2 | 10.0 | 243/131 | 26 | 243/215 | 15 | 85 | 10 |
| Fensulfothion | OPPs | C11H17O4PS2 | 8.5 | 309/252.9 | 24 | 309/280.9 | 18 | 120 | 12 |
| Etrimfos | OPPs | C10H17N2O4PS | 12.4 | 293.1/265 | 21 | 293.1/124.7 | 32 | 110 | 13 |
| Fenamiphos | OPPs | C13H22NO3PS | 9.8 | 304.2/216.8 | 30 | 304.2/233.9 | 22 | 120 | 12 |
| Fenitrothion | OPPs | C9H12NO5PS | 11.6 | 278.2/125 | 28 | 278.2/246.2 | 23 | 89 | 8 |
| Fensulfothion | OPPs | C11H17O4PS2 | 8.5 | 309/252.9 | 24 | 309/280.9 | 18 | 120 | 12 |
| Fenthion | OPPs | C10H15O3PS2 | 12.8 | 279.1/168.8 | 23 | 279.1/246.9 | 17 | 111 | 12 |
| Fonophos | OPPs | C10H15OPS2 | 13.2 | 246.9/109 | 24 | 246.9/136.9 | 14 | 80 | 10 |
| Isazophos | OPPs | C9H17ClN3O3PS | 11.7 | 314/119.9 | 36 | 314/119.9 | 22 | 81 | 10 |
| Isocarbophos | OPPs | C11H16NO4PS | 9.6 | 290.3/231 | 18 | 290.3/121 | 36 | 70 | 10 |
| Isofenphos-methyl | OPPs | C14H22NO4PS | 13.0 | 332.1/231 | 17 | 332.1/273 | 7 | 70 | 12 |
| Malaoxon | OPPs | C10H19O7PS | 7.6 | 315.1/99 | 32 | 315.1/127.1 | 17 | 75 | 13 |
| Malathion | OPPs | C10H19O6PS2 | 11.1 | 331.1/126.9 | 17 | 331.1/284.8 | 9 | 110 | 10 |
| Methacrifos | OPPs | C7H13O5PS | 9.8 | 241/208.9 | 12 | 241/124.9 | 20 | 100 | 6 |
| Methamidophos | OPPs | C2H8NO2PS | 1.3 | 142/93.9 | 17 | 142/124.9 | 16 | 80 | 12 |
| Methidathion | OPPs | C6H11N2O4PS3 | 9.8 | 303/145 | 12 | 303/85 | 27 | 104 | 12 |
| Mevinphos | OPPs | C7H13O6P | 6.6 | 225.1/126.9 | 19 | 225.1/193.1 | 8 | 85 | 12 |
| Monocrotophos | OPPs | C7H14NO5P | 5.5 | 224/192.9 | 11 | 224/126.9 | 18 | 95 | 11 |
| Omethoate | OPPs | C5H12NO4PS | 5.0 | 214/183 | 14 | 214/154.9 | 20 | 92 | 6 |
| Parathion | OPPs | C10H14NO5PS | 12.8 | 292.2/235.9 | 18 | 292.2/264 | 13 | 60 | 11 |
| Phenthoate | OPPs | C12H17O4PS2 | 12.8 | 321.1/247 | 14 | 321.1/163 | 14 | 71 | 11 |
| Phorate | OPPs | C7H17O2PS3 | 13.4 | 261.1/74.9 | 15 | 261.1/198.9 | 11 | 64 | 7 |
| Phorate sulfone | OPPs | C7H17O4PS3 | 9.5 | 293/171 | 15 | 293/247 | 9 | 102 | 11 |
| Phorate sulfoxide | OPPs | C7H17O4PS2 | 8.0 | 277/199.1 | 13 | 277/142.9 | 27 | 85 | 11 |
| Phosalone | OPPs | C12H15ClNO4PS2 | 13.8 | 368.2/182 | 22 | 368.2/322.1 | 14 | 108 | 12 |
| Phosfolan | OPPs | C7H14NO3PS2 | 6.6 | 256.1/140 | 32 | 256.1/228 | 18 | 82 | 12 |
| Phosmet | OPPs | C11H12NO4PS2 | 10.3 | 318.1/160.2 | 17 | 318.1/133.1 | 48 | 108 | 10 |
| Phosphamidon | OPPs | C10H19ClNO5P | 6.9 | 300.1/174 | 17 | 300.1/127 | 27 | 120 | 13 |
| Phoxim | OPPs | C12H15N2O3PS | 13.7 | 299.1/76.9 | 40 | 299.1/129.1 | 16 | 81 | 12 |
| Pirimiphos ethyl | OPPs | C13H24N3O3PS | 13.6 | 334.1/198.1 | 30 | 334.1/182.1 | 30 | 89 | 9 |
| Pirimiphos-methyl | OPPs | C11H20N3O3PS | 11.6 | 306.1/164.1 | 30 | 306.1/108 | 38 | 110 | 8 |
| Profenofos | OPPs | C11H15BrClO3PS | 13.7 | 373.1/302.9 | 24 | 373.1/344.7 | 18 | 134 | 12 |
| Propetamphos | OPPs | C10H20NO4PS | 11.5 | 282.1/137.9 | 23 | 282.1/156 | 18 | 122 | 6 |
| Quinalphos | OPPs | C12H15N2O3PS | 12.2 | 299.1/162.9 | 31 | 299.1/146.9 | 32 | 82 | 10 |
| Sulfotep | OPPs | C8H20O5P2S2 | 13.2 | 323.1/170.9 | 20 | 323.1/295 | 13 | 118 | 9 |
| Terbufos | OPPs | C9H21O2PS3 | 15.1 | 289/102.9 | 10 | 289/232.9 | 8 | 84 | 13 |
| Tetrachlorvinphos | OPPs | C10H9Cl4O4P | 11.0 | 365.1/127.1 | 16 | 365.1/239 | 29 | 124 | 11 |
| Triazophos | OPPs | C12H16N3O3PS | 11.4 | 314.1/162 | 23 | 314.1/286 | 16 | 97 | 9 |
| Chlorophos | OPPs | C4H8Cl3O4P | 5.9 | 257.1/109.1 | 22 | 257.1/220.8 | 15 | 111 | 10 |
| Ditalimfos | OPPs | C12H14NO4PS | 11.4 | 300.1/148.1 | 23 | 300.1/244 | 17 | 101 | 11 |
| Iprobenfos | OPPs | C13H21O3PS | 10.6 | 289.1/90.9 | 26 | 289.1/205 | 15 | 100 | 11 |
| Pyrazophos | OPPs | C14H20N3O5PS | 12.7 | 374.2/222 | 28 | 374.2/194.1 | 43 | 76 | 11 |
| Tolclofos-methyl | OPPs | C9H11Cl2O3PS | 13.6 | 301.1/269 | 20 | 301.1/124.9 | 22 | 107 | 11 |
| Bitertanol | TIZs | C20H23N3O2 | 10.9 | 338.3/99 | 19 | 338.3/269 | 12 | 93 | 10 |
| Difenoconazole | TIZs | C19H17Cl2N3O3 | 12.1 | 406/251 | 29 | 406/337 | 24 | 91 | 11 |
| Diniconazole | TIZs | C15H17Cl2N3O | 11.6 | 326.1/70 | 26 | 326.1/158.9 | 34 | 97 | 12 |
| Flusilazole | TIZs | C16H15F2N3Si | 10.6 | 316.1/165.1 | 34 | 316.1/247 | 23 | 127 | 13 |
| Flutriafol | TIZs | C16H13F2N3O | 8.0 | 302.1/70 | 21 | 302.1/123 | 35 | 85 | 12 |
| Hexaconazole | TIZs | C14H17Cl2N3O | 10.7 | 314/70 | 22 | 314/159 | 38 | 120 | 10 |
| Myclobutanil | TIZs | C15H17ClN4 | 9.9 | 289/70 | 21 | 289/125 | 41 | 141 | 9 |
| Penconazole | TIZs | C13H15Cl2N3 | 10.7 | 284.1/69.9 | 23 | 284.1/158.9 | 40 | 105 | 9 |
| Propiconazole | TIZs | C15H17Cl2N3O2 | 11.0 | 342.1/158.9 | 35 | 342.1/69 | 24 | 95 | 13 |
| Tebuconazole | TIZs | C16H22ClN3O | 10.4 | 308.2/70 | 23 | 308.2/124.9 | 46 | 141 | 8 |
| Triadimefon | TIZs | C14H16ClN3O2 | 10.2 | 294/197 | 19 | 294/68.9 | 26 | 114 | 9 |
| Triadimenol | TIZs | C14H18ClN3O2 | 9.1 | 296/70 | 15 | 296/99.1 | 20 | 64 | 12 |
| Acetamiprid | Neonicotinoids | C10H11ClN4 | 6.5 | 223/126 | 25 | 223/56.1 | 22 | 110 | 11 |
| Imidacloprid | Neonicotinoids | C9H10ClN5O2 | 6.4 | 256.1/209 | 25 | 256.1/175 | 27 | 96 | 9 |
| Pymetrozine | Pyridines | C10H11N5O | 1.3 | 218/105 | 30 | 218/78.9 | 47 | 89 | 13 |
| Tebufenozide | Others | C22H28N2O2 | 12.1 | 353.2/132.9 | 9 | 353.2/297.1 | 21 | 98 | 11 |
| Azoxystrobin | Others | C22H17N3O5 | 10.2 | 404.1/372 | 21 | 404.1/372 | 32 | 108 | 10 |
| Isoprothiolane | Others | C12H18O4S2 | 11.0 | 291/188.9 | 27 | 291/230.9 | 14 | 81 | 11 |
| Metalaxyl | Anilines | C15H21NO4 | 8.0 | 280/220 | 17 | 280/192.1 | 24 | 118 | 11 |
| Triflumizole | Imidazoles | C15H15ClF3N3O | 9.9 | 346.1/278 | 14 | 346.1/73 | 21 | 64 | 11 |
| Fenpropathrin | PYHs | C22H23NO3 | 15.8 | 350.1/125.1 | 20 | 350.1/97 | 41 | 130 | 10 |
3.2 Optimization of LC conditions
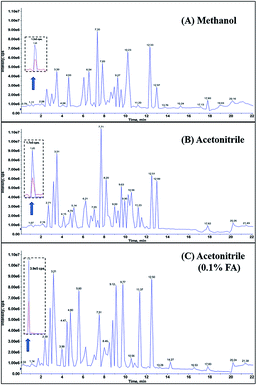
By application of the optimal gradient program, it took only 22 min to simultaneously determine the 104 pesticides in a single run. Methanol and acetonitrile, the most common components of reversed mobile phases, generally provide different sensitivity and selectivity for the compounds in LC analysis. In our investigation, the majority of the peaks showed superior chromatographic resolution and higher detected signal in acetonitrile compared to those in methanol (Fig. 1). In addition, the peak shapes for pesticides such as methamidophos, thiabendazole, and isoprothiolane were markedly improved with acetonitrile as the mobile phase. The volatile formic acid not only contributed to promoting protonation of the targeted compounds, but also suppressed the ionization of residual silanols in the stationary phase and thus improved the peak shapes. Using acetonitrile containing 0.1% formic acid (v/v), the intensities of most of the pesticide peaks are greatly enhanced (Fig. 1). In some cases, there was a deterioration in the detected intensity, for instance with methomyl. All things considered, acetonitrile containing 0.1% formic acid was chosen.3.3 MWNTs-based QuEChERS procedure
In spite of the superior chromatographic resolution and sensitivity achieved with the LC-QqLIT-MS/MS system, the instrument is readily contaminated by interfering components from the complicated matrix, and this leads to a decrease in detection performance together with high maintenance costs. Sample pre-treatment including QuEChERS may be essential. Acetonitrile was chosen as the extraction solvent on account of the good solubility of analytes of a wide polarity range therein and its compatibility with LC-MS/MS. The extraction efficiencies were closely related to the volume of solvent. Hence, a fortified powder sample (1.0 g) was infiltrated with deionized water and extracted with 5 mL, 10 mL, or 20 mL acetonitrile in the initial extraction process. The results (Fig. 2A) showed that an increase in the amount of acetonitrile generated an incremental extraction ratio in the range 70–120%. However, excess solvent (20 mL) not only increased the likelihood of co-extracts that could be detrimental to the subsequent analysis, but also brought about the consumption of a hazardous substance. Hence, 10 mL of acetonitrile was used in the extraction step.To evaluate the capacity for removing impurities in dried tangerine peel, sorbents based on PSA, MWNTs, and GCB were investigated separately or in combination in a preliminary analysis. Dried tangerine peel contains abundant essential oils, organic acids, flavonoids, sugars, and carotenoids (natural pigments).33–36 To decrease the interfering effects of these, combinations of PSA with either MWNTs or GCB were found to provide better clean-up performances. In order to achieve satisfactory recoveries, different compositions of sorbents provided in Table 2 were tested for three times. The mean recoveries of pesticides after the clean-up of MWNTs group and CNT group were compared by a paring t-test, the difference (P < 0.05) indicated a statistical significance between the two groups. The effects of MWNTs on representative compounds are shown in Fig. 2B; multi-class pesticides showed acceptable recoveries using composition (4). The results indicated that MWNTs as an alternative gave good recoveries, and 10 mg led to more than 90% of the pesticides showing the required recoveries between 70% and 120%, possibly due to the merits of superior large surface area and the π–π interaction between carotenoids and MWNTs. Excess MWNTs, however, led to a great loss of recovery for analytes with a planar structure, such as azoxystrobin, thiabendazole, and carbendazim. Finally, the composition of 25 mg PSA, 10 mg MWNTs, and 150 mg anh. MgSO4 was chosen as the optimal one for clean-up.
| Absorbents | Group number | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| a Note: anh. MgSO4, anhydrous magnesium sulfate; PSA, primary secondary amine; GCB, graphitized carbon black; MWNTs, multi-walled carbon nanotubes. | ||||||
| Anh. MgSO4 (mg) | 150 | 150 | 150 | 150 | 150 | 150 |
| PSA (mg) | 25 | 25 | 25 | 25 | 25 | 25 |
| GCB (mg) | 5 | — | 10 | — | 15 | — |
| MWNTs (mg) | — | 5 | — | 10 | — | 15 |
3.4 Method validation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. The LOQs and LODs in the method were in the ranges 0.5–100 μg kg−1 and 0.2–40 μg kg−1, respectively, which met the detection requirements of trace pesticides in dried tangerine peel referring to MRLs (0.01–15 mg kg−1) stipulated for tangerine by the European Commission.
1, respectively. The LOQs and LODs in the method were in the ranges 0.5–100 μg kg−1 and 0.2–40 μg kg−1, respectively, which met the detection requirements of trace pesticides in dried tangerine peel referring to MRLs (0.01–15 mg kg−1) stipulated for tangerine by the European Commission. | ||
| Fig. 4 Scheduled MRM chromatograms of (a) 104 standard mixtures at 10 ng mL−1 in solvent and (b) blank matrix extract of dried tangerine peel. | ||
To further validate the robustness of the MWNTs-based QuEChERS method in relation to dried tangerine peel, method recovery was performed with fortified samples at four concentration levels of 10 μg kg−1, 50 μg kg−1, 100 μg kg−1, and 500 μg kg−1 in triplicate, covering the range of EU2 and Chinese38 MRLs for pesticides. The results obtained are included in Table 2; the average recoveries at each concentration were in the ranges 68.0–117.2% with RSDs of 1.4–18.1%, 69.8–117.6% with RSDs of 1.5–16.9%, 74.7–110.7% with RSDs of 1.6–15.2%, and 70.8–116.4% with RSDs of 2.2–17.2%. On the whole, the majority of recoveries fell in the range 71.1–117.6% (90.6% on average), except for some pesticides such as thiabendazole, teflubenzuron, hexaflumuron, and methomyl (63.9–69.8%), which could nevertheless also be accepted.
The intra-day precision was assessed by repeatedly injecting a spiked sample solution at 10 ng mL−1 six times, and inter-day precision was measured at the same concentration on three successive days. The RSD values for all the experiments were within 18.9%, thus meeting the EU criterion (RSDs of 20% for precision), and demonstrated good repeatability by the described method.
3.5 Results of real samples and EPI spectra confirmation
To demonstrate the feasibility of the proposed method in routine analysis, it was applied to 57 dried tangerine peel samples, which were randomly selected from drugstores or medicine markets across China. After the optimized MWNTs-based QuEChERS treatment, all samples were analyzed on the system of UFLC coupled with scheduled MRM scan along with synchronous triggering of the acquisition of fragment-rich EPI spectra. The spiked samples, standard solution, and blank matrix were prepared in advance to provide references for the subsequent identification. The analytes were identified by retention time, characteristic ion transitions of the most intense product ion (Q) and secondary ion (q), together with the product ion abundance ratios (Q/q) matching those of standards within 20% relative deviation. To decrease the false-positive results, most of the pesticides were confirmed by comparison of their characteristic fragment ions in a positive sample with those of standards. For example, carbendazim produced prominent fragments at m/z 159.8 ([M + H − CH3OH]+) and m/z 131.8 ([M + H − CH3OH − CO]+) as well as other low intensity ions; the same fragments were observed in a contaminated sample, which revealed the usefulness of the current method with synchronous EPI spectra. For the relevant pesticides, the contamination levels in real samples were quantified. The results of the determination are summarized in Table 3, and typical chromatograms and mass spectra are presented in Fig. 5.| Pesticide | Number of incurred samples/ratio | Detected range/mg kg−1 | Beyond number/ratio | WHO class** | MRL***/mg kg−1 |
|---|---|---|---|---|---|
| a Note: *, the detected level exceeded the linearity range was diluted with matrix extract; **, (Ib = highly hazardous; II = moderately hazardous; III = slightly hazardous; U = unlikely to present acute hazard in normal use); ***, Maximum Residue Limit (MRL), Part A of Annex I to Reg. 396/2005; LOQ, the limit of quantification. | |||||
| Carbendazim | 26(45.6%) | 0.019–4.71* | 4(28.1%) | U | 0.2 |
| Thiophanate-methyl | 42(73.7%) | <LOQ–3.99* | — | U | 6 |
| Chlorpyrifos | 15(26.3%) | 0.066–0.863 | 7(12.3%) | II | 0.3 |
| Carbofuran | 20(35.1%) | <LOQ–0.030 | — | Ib | 0.5 |
| Malathion | 32(57.9%) | <LOQ–0.074 | — | III | 2 |
| Methidathion | 25(43.9%) | 0.033–0.635 | 25(43.9%) | Ib | 0.02 |
| Acetamiprid | 23(40.4%) | <LOQ–0.039 | — | — | 0.9 |
| Imidacloprid | 16(28.1%) | <LOQ–0.096 | — | II | 1 |
| Difenoconazole | 20(35.1%) | 0.028–0.080 | — | II | 0.1 |
| Fenpropathrin | 2(3.51%) | 0.035–0.048 | — | II | 2 |
| Triazolone | 1(1.75%) | 0.066 | — | II | 0.1 |
| Isoprothiolane | 3(5.26%) | 0.025–0.046 | 3(5.26%) | II | 0.01 |
| Iprobenfos | 2(3.51%) | <LOQ–0.025 | II | — | |
| Phenthoate | 5(8.77%) | 0.027–0.081 | — | II | — |
| Isocarbophos | 3(5.26%) | 0.032–0.093 | — | — | — |
| Isoprocarb | 2(3.51%) | <LOQ | — | II | — |
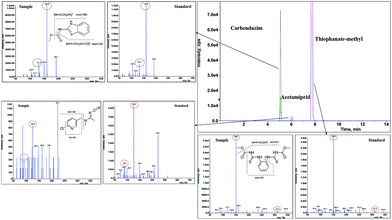 | ||
| Fig. 5 Typical selective ion chromatograms and EPI spectra for confirmation of pesticides in positive dried tangerine peel. | ||
A total of 16 of the 104 compounds were detected in the real dried tangerine peel samples. Considering that peel constitutes a raw material derived from tangerines, and there is no specific MRL for pesticides in the former, the MRL values refer to tangerine. Most of the pesticides found in positive samples were below the MRLs, except for carbendazim (28.1%), chlorpyrifos (12.3%), methidathion (43.9%) and isoprothiolane (5.26%). In China, the compounds such as chlorpyrifos, methidathion, thiophanate-methyl etc. are authorized for use in tangerine. However, they must be applied on the basis of agronomic prescription. The frequent occurrence of thiophanate-methyl (73.7%), malathion (57.9%) and methidathion (43.9%) residues revealed their overdose and abuse in tangerine cultivation. Especially for methidathion, which may be classified as highly hazardous,39 greater attention needs to be paid to the development of detection methods as well as good agricultural practice in tangerine cultivation. The results obtained in the present study are similar to those reported by Golge et al.40 and Bakırcı et al.,41 showing that tangerine and its peel are easily contaminated by the insecticide chlorpyrifos. The levels of some compounds found in this study are inconsistent with those reported by Blasco et al.,42 such as carbendazim and imidacloprid at 51.9% and 9.6%, respectively. Probably because the non-systemic insecticide works on the surface of the plant, the occurrence of methidathion residue in fresh tangerine fruits is 32.6%, as compared to 43.9% in dried tangerine peels. Other pesticides, such as acetamiprid, carbofuran, difenoconazole and imidacloprid were found at high frequencies of 40.4%, 35.1%, 35.1% and 28.1%, respectively, but none of their residue levels were beyond their MRLs in fresh tangerine. In view of the wide application of the dried tangerine peels but high contamination with multi-pesticides, it reminds us that the MRL standards in dried tangerine peels are urgently demanded to be established for the human health. Unlike the fresh fruit, the dried peel is processed by washing, peeling, drying and storing. Hence, the standards of pesticide residues in dried tangerine peel should take the procession into consideration as well as dietary habits of human.
4 Conclusions
In summary, a combination of MWNTs and PSA has been developed as an excellent sorbent for use in a QuEChERS method and validated as a rapid, efficient, and high-recovery pre-treatment measure for multiclass pesticide residues in dried tangerine peel. MWNTs proved to be a good option, not only because of their superior adsorption capacity, but also because of their abundant source, making them inexpensive. By optimizing the volume of acetonitrile and the absorbent package (MWNTs/PSA/MgSO4), the clean-up procedure led to decreased interference from co-extracts and improved matrix effects (63.5% for ME within 0.8–1.2) and recovery (90.6% on average). LC-MS/MS has been demonstrated as a simple, sensitive, and high-throughput analytical method for the simultaneous screening of 104 pesticides in real samples, and methodology validation results meet the criteria of SANCO/12495/2011. Applying the proposed method to 57 dried tangerine peel samples, 16 pesticides were identified, and the residue levels of carbendazim, chlorpyrifos, methidathion and isoprothiolane in some dried tangerine peel samples exceeded their MRLs. In addition, EPI spectra provided a useful tool to confirm the positive results. Therefore, we have developed a MWNTs-based QuEChERS pre-treatment and EPI spectra synchronous LC-MS/MS analytical method that offers a significant improvement in analytical performance in relation to complex matrices for routine pesticide screening.Acknowledgements
The authors are very grateful for powerful suggestions from anonymous referees. We also appreciate National Natural Science Foundation of China (81274072, 81473346), PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (No. 33320140146), Xiehe New Star Project and Specialized Research Fund for Major Science and Technology Project of Hainan, China (ZDZX2013008).References
- Z. Yi, Y. Yu, Y. Liang and B. Zeng, LWT-Food Sci. Technol., 2008, 41, 597–603 CrossRef CAS PubMed
.
- Regulation (EC) No. 396/2005 of the european parliament and of the council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending council directive 91/414/EEC.
- E. Commission, EU pesticides database, http://ec.europa.eu/sanco_pesticides/public/index.cfm.
- Y. Wang, H. Y. Jin, S. C. Ma, J. Lu and R. C. Lin, J. Chromatogr. A, 2011, 1218, 334–342 CrossRef CAS PubMed
.
- C. Lourencetti, M. R. R. de Marchi and M. L. Ribeiro, Talanta, 2008, 77, 701–709 CrossRef CAS PubMed
.
- Z. Shi, J. Hu, Q. Li, S. Zhang, Y. Liang and H. Zhang, J. Chromatogr., A, 2014, 1355, 219–227 CrossRef CAS PubMed
.
- Y. Li, R. A. Kelley, T. D. Anderson and M. J. Lydy, Talanta, 2015, 140, 81–87 CrossRef CAS PubMed
.
- J. Li, D. Liu, T. Wu, W. Zhao, Z. Zhou and P. Wang, Food Chem., 2014, 151, 47–52 CrossRef CAS PubMed
.
- M. Ishibashi, T. Ando, M. Sakai, A. Matsubara, T. Uchikata, E. Fukusaki and T. Bamba, J. Chromatogr., A, 2012, 1266, 143–148 CrossRef CAS PubMed
.
- Y. Ke, F. Zhu, F. Zeng, T. Luan, C. Su and G. Ouyang, J. Chromatogr., A, 2013, 1300, 187–192 CrossRef CAS PubMed
.
- R. C. Duca, G. Salquebre, E. Hardy and B. M. R. Appenzeller, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 955–956, 98–107 CrossRef CAS PubMed
.
- R. Xu, J. Wu, Y. Liu, R. Zhao, B. Chen, M. Yang and J. Chen, Chemosphere, 2011, 84, 908–912 CrossRef CAS PubMed
.
- S. Walorczyk, Talanta, 2014, 120, 106–113 CrossRef CAS PubMed
.
- G. Du, Y. Song and Y. Wang, J. Sep. Sci., 2011, 34, 3372–3382 CrossRef CAS PubMed
.
- L. Chen, F. Song, Z. Liu, Z. Zheng, J. Xing and S. Liu, J. Chromatogr., A, 2012, 1225, 132–140 CrossRef CAS PubMed
.
- M. Trojanowicz, TrAC, Trends Anal. Chem., 2006, 25, 480–489 CrossRef CAS PubMed
.
- L. M. Ravelo-Pérez, J. Hernández-Borges and M. Á. Rodríguez-Delgado, J. Chromatogr., A, 2008, 1211, 33–42 CrossRef PubMed
.
- Z. Yu, Z. Qin, H. Ji, X. Du, Y. Chen, P. Pan, H. Wang and Y. Liu, Chromatographia, 2010, 72, 1073–1081 CAS
.
- Q. Zhou, J. Xiao, G. Xie, W. Wang, Y. Ding and H. Bai, Microchim. Acta, 2009, 164, 419–424 CrossRef CAS
.
- S. Dahane, M. Gil García, A. Uclés Moreno, M. Martínez Galera, M. D. Socías Viciana and A. Derdour, Microchim. Acta, 2015, 182, 95–103 CrossRef CAS
.
- G. Fang, G. Min, J. He, C. Zhang, K. Qian and S. Wang, J. Agric. Food Chem., 2009, 57, 3040–3045 CrossRef CAS PubMed
.
- S. Guan, Z. Yu, H. Yu, C. Song, Z. Song and Z. Qin, Chromatographia, 2011, 73, 33–41 CAS
.
- M. González-Curbelo, M. Asensio-Ramos, A. Herrera-Herrera and J. Hernández-Borges, Anal. Bioanal. Chem., 2012, 404, 183–196 CrossRef PubMed
.
- P. Zhao, L. Wang, J. Luo, J. Li and C. Pan, J. Sep. Sci., 2012, 35, 153–158 CrossRef CAS PubMed
.
- X. Hou, S. Lei, S. Qiu, L. Guo, S. Yi and W. Liu, Food Chem., 2014, 153, 121–129 CrossRef CAS PubMed
.
- P. Zhao, L. Wang, Y. Jiang, F. Zhang and C. Pan, J. Agric. Food Chem., 2012, 60, 4026–4033 CrossRef CAS PubMed
.
- L. Alder, K. Greulich, G. Kempe and B. Vieth, Mass Spectrom. Rev., 2006, 25, 838–865 CrossRef CAS PubMed
.
- R. Mercadante, E. Polledri, S. Scurati, A. Moretto and S. Fustinoni, Chem. Res. Toxicol., 2014, 27, 1943–1949 CrossRef CAS PubMed
.
- K. L. Lynch, A. R. Breaud, H. Vandenberghe, A. H. B. Wu and W. Clarke, Clin. Chim. Acta, 2010, 411, 1474–1481 CrossRef CAS PubMed
.
- X. Li, T. M. Kamenecka and M. D. Cameron, Chem. Res. Toxicol., 2009, 22, 1736–1742 CrossRef CAS PubMed
.
- F. Li, Y. Hsieh, L. Kang, C. Sondey, J. Lachowicz and W. A. Korfmacher, Bioanalysis, 2009, 1, 299–307 CrossRef CAS PubMed
.
- H. Lee and B. J. Lee, Food Addit. Contam., Part A, 2011, 28, 396–407 CrossRef CAS PubMed
.
- G. Zheng, D. Yang, D. Wang, F. Zhou, X. Yang and L. Jiang, J. Agric. Food Chem., 2009, 57, 6552–6557 CrossRef CAS PubMed
.
- D. Wang, J. Wang, X. Huang, Y. Tu and K. Ni, J. Pharm. Biomed. Anal., 2007, 44, 63–69 CrossRef CAS PubMed
.
- Y. Wang, L. Yi, Y. Liang, H. Li, D. Yuan, H. Gao and M. Zeng, J. Pharm. Biomed. Anal., 2008, 46, 66–74 CrossRef CAS PubMed
.
- Y. C. Wang, Y. C. Chuang and H. W. Hsu, Food Chem., 2008, 106, 277–284 CrossRef CAS PubMed
.
- E. Commission, Document no. SANCO/12495/2011, Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed, 2011, p. 11 Search PubMed
.
- GB/T 2763-2014, National food safety standard-maximum residue limits for pesticides in food.
- W. H. Organization, WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009, 2010 Search PubMed
.
- O. Golge and B. Kabak, J. Food Compos. Anal., 2015, 41, 86–97 CrossRef CAS PubMed
.
- G. T. Bakırcı, D. B. Yaman Acay, F. Bakırcı and S. Ötleş, Food Chem., 2014, 160, 379–392 CrossRef PubMed
.
- C. Blasco, G. Font and Y. Picó, Food Control, 2006, 17, 841–846 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15348d |
| This journal is © The Royal Society of Chemistry 2015 |