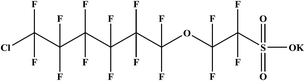
Mechanochemical destruction of a chlorinated polyfluorinated ether sulfonate (F-53B, a PFOS alternative) assisted by sodium persulfate†
Xue Yana,
Xitao Liu*a,
Chengdu Qia,
Dali Wangb and
Chunye Lina
aState Key Laboratory of Water Environment Simulation, School of Environment, Beijing Normal University, Beijing 100875, China. E-mail: liuxt@bnu.edu.cn; Fax: +86-10-58807743; Tel: +86-10-58805080
bCalcium Carbide Factory, Jilin Petrochemical Company, Petro China, Jilin 132000, China
First published on 6th October 2015
Abstract
A chlorinated polyfluorinated ether sulfonate (F-53B, C8ClF16O4SK) has been widely used as a mist suppressant by the chrome plating industry in China for more than 30 years. It was reported to be moderately toxic and as resistant to degradation as perfluorooctane sulfonate (PFOS). Considering the need for safe disposal of wastes containing F-53B, fast and effective methods are urgently required, of which the mechanochemical destruction (MCD) method seems to be a good alternative. In the present study, sodium persulfate (PS) was tested as a novel co-milling reagent in the MCD process for the degradation of F-53B. F-53B, PS and sodium hydroxide (NaOH) were co-ground in a planetary ball mill, and the ground samples were analyzed by liquid chromatography-mass spectrometry (LC-MS), ion chromatography (IC) and a total organic carbon (TOC) analyzer. Fourier transform infrared spectroscopy (FTIR), X-ray powder diffraction (XRD) and X-ray photoelectron spectrometry (XPS) analyses were conducted as assistant measures. The results revealed that after 8 h of milling with 60 g balls at the best mass ratio (PS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH
NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F-53B = 4.17
F-53B = 4.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.75
1.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05), 88% of F-53B was destroyed, the fluoride recovery efficiency was 54%, and the destruction efficiency closely related to the milling time. TOC analysis showed considerable mineralization of F-53B. Overall, MCD was suggested to be a promising method in the degradation of recalcitrant chemicals like F-53B.
0.05), 88% of F-53B was destroyed, the fluoride recovery efficiency was 54%, and the destruction efficiency closely related to the milling time. TOC analysis showed considerable mineralization of F-53B. Overall, MCD was suggested to be a promising method in the degradation of recalcitrant chemicals like F-53B.
1. Introduction
Over the past several decades, perfluorinated compounds (PFCs) have been extensively used as firefighting foams, lubricants, feroclean and other industrial and consumer products.1–3 Among them, a chlorinated polyfluorinated ether sulfonate (F-53B, Table 1) has been widely employed as a chromium fog inhibitor for more than 30 years in the chrome plating industry in China. It has been detected in surface water polluted by the wastes from the chrome plating industry.4 Besides, Ruan et al. studied sewage sludge samples collected from individual wastewater treatment plants across China and the geometric mean concentration of F-53B was found to be 2.15 ng g−1 (the maximum concentration was 209 ng g−1).5 Enough attention must be paid to F-53B due to its toxicity, increasing demand and the potential hazards to the environment.4The sound destruction of PFCs wastes is becoming a challenging issue. Nowadays, photochemical, sonochemical and electrochemical approaches have been exploited for the degradation of PFCs.6–8 However, these methods usually need harsh reaction conditions or the destruction of PFCs is not complete;9 besides, these methods mainly focus on aqueous system and are restricted for the treatment of the solid wastes. For the treatment of solid wastes, incineration technology could be a better choice, but the generation of toxic by-products, such as dioxins, is a real hassle.10 Mechanochemical destruction (MCD) has been successfully applied to the decomposition of various halogenated organic pollutants and has been proved to be a promising non-combustion approach, with various merits over the conventional approaches.
The most commonly used device in MCD is a planetary ball mill, in which the mixtures of harmful wastes and proper additives are put in along with milling balls. During the milling process, the device rotates at a high speed, and transient high temperature or even triboplasmas can be created by the mechanic force such as impact, fraction, stress and deformation,11,12 which play a significant role in the reaction between contaminants and reagents. Compared with conventional combustion approaches, MCD does not need heating and produces less wastes, which not only saves energy, but also reduces the generation of toxic products.10,12,13 What's more, MCD has been proven to be a promising non-combustion technology for the disposal of halogenated organic pollutants, such as tetrabromobisphenol A, mirex, perfluorooctane sulfonate (PFOS) and so on.11,12,14–16
Persulfate (PS) has been given rising attention due to its stability and oxidbillity in the recent years. Furthermore, the generated sulfate radical (SO4˙−) and hydroxyl radical (˙OH) under the activation of heat/UV, metal (usually Fe2+) and alkali (eqn (1)–(3)), are more effective for the degradation of organic contaminants.17–19 If PS is used as a co-milling reagent in the MCD process, PS maybe activated by mechanical force and heat during the milling process, and sulfate radical can be generated in the system. Besides, as an activator, sodium hydroxide (NaOH) can induce the generation of ˙OH. So, in the present study, F-53B was co-ground with a mixture of PS and NaOH in a planetary ball mill at room temperature to investigate the effectiveness of the PS-assisted MCD process. The degradation efficiency was evaluated by changing the mass ratio of PS and NaOH. Moreover, characterization of the milled mixture was carried out in order to verify the variations of chemical bonds and functional groups. Finally, possible reaction pathways and degradation mechanism were proposed.
| S2O82− + UV/heat → 2SO4˙−, k = 5.7 × 10−5 s−1 | (1) |
| S2O82− + Fe2+ → SO4˙− + SO42− + Fe3+, k = 20 M−1 s−1 | (2) |
| 2S2O82− + 4OH− → SO4˙− + O2˙− + 3SO42− + 2H2O | (3) |
2. Materials and methods
2.1 Materials
F-53B (>98%) was purchased from Shanghai Synica Co., Ltd (Shanghai, China). Methanol was obtained from J&K Scientific (Beijing, China). Sodium persulfate (Na2S2O8), sodium hydroxide (NaOH), sodium sulfate (Na2SO4), sodium fluoride (NaF) and other inorganic reagents were provided by Sinopharm (Beijing, China). All chemical reagents and organic solvents were at least analytical grade and were used as received without further purification. Solutions were prepared with Milli-Q deionized water (DI, 18.2 MΩ cm).2.2 Ball milling procedures
A planetary ball mill (QM-3SP2, Nanjing University Instrument Corporation, China) equipped with four zirconia milling pots (100 mL) was used in the experiment. Co-milling reagents (PS, NaOH, PS–FeSO4·7H2O, PS–NaOH) were mixed with 0.05 g of F-53B and then put into the pots along with 60 g zirconia balls with diameter of 9.6 mm. The rotation speed was set at 275 rpm and the interval time was 5 min after every 30 min of operation in case of overheat. The milled samples were all collected and preserved in dry and hermetic apparatus for further analysis.2.3 Sample analysis
For F-53B analysis, 0.2 g of each milled sample was dissolved in 10 mL methanol with 30 min ultrasonic (US) treatment at 60 °C (Text S1†). After that, the extract was centrifuged at 3000 rpm for 10 min and the solution was filtered by 0.22 μm polyether sulfone (PES) filter. The separated solid residue was extracted one more time in the same manner and the mixture of the solutions was subjected to instrumental analysis. F-53B was measured by liquid chromatography-mass spectrometry (LC-MS). Separation was done by an Ultimate 3000 HPLC system (Dionex, Germering, Germany) equipped with a Zorbax Eclipse Plus C18 column (150 mm × 4.6 mm, 5 μm, Agilent, Santa Clara, CA, USA). Target compound was detected by an API 3200 triple quadruple mass spectrometer (Applied Biosystems, Foster City, USA) in the negative mode of ionization. A 10 μL volume was injected by the autosample system. The flow rate of mobile phase was 1 mL min−1 and the column unit was held at 30 °C. Methanol/10 mM ammonium acetate (40/60, v/v) was used as initial mobile phase and held for 1 min, then methanol was increased to 100% gradually during the next 6 min and kept for 3.5 min, followed by equilibrium at 40% methanol for 2.5 min. The ionization was set at an ion spray voltage of −4.5 kV and at a temperature of 450 °C, using nitrogen for drying. The flows of curtain gas, collision gas, ion source gas 1 and ion source gas 2 were set at 20, 5, 30, and 60 psi, respectively.For ions, PS and TOC analysis, 0.2 g of each sample was extracted by ultrapure water in the same manner as above. Concentrations of fluorine ion (F−) and sulfate ion (SO42−) were analyzed with an ion chromatography system (ICS-1100, Dionex, USA) equipped with a guard column and a separation column (DionexIonPacAS19 Analytical, 4 mm × 250 mm, USA). The injection volume was 20 μL and the column temperature was held at 35 °C. The mobile phase was 20 mM KOH and the flow rate was 1 mL min−1. The defluorination efficiency can be expressed as (measured soluble fluoride ion)/(calculated fluorine from initial F-53B). The concentration of PS was measured by a spectrophotometric method using potassium iodide.20 The total organic carbon (TOC) concentration was detected by a TOC analyzer (TOC-L CPN, Shimadzu, Japan), which is based on a combustion catalytic oxidation method, using a highly sensitive multichannel nondispersive infrared detector (NDIR).
2.4 Characterization of the milled sample
The milled mixture collected at specific time was characterized by various approaches. X-ray powder diffraction (XRD) was used to identify the crystalline products, which was conducted using an XRD (X' pert pro MPD, PANalytical, Holland) with Cu Kα radiation at a speed of 6° min−1 ranging from of 2θ = 20–80°. Fourier transform infrared (FTIR) was carried out using Nicolet 6700 (NEXUS 670, East branch instrument, America) with the KBr disk method from 250 to 4250 cm−1. X-ray photoelectron spectrometry (XPS) was conducted using Scanning X-ray Microprobe (ESCSLAB 250Xi, ThermoFisher, America), in which the binding energy was calibrated using the C (1s) peak at 284.6 eV. Raman spectrometer (Lab RAMAramis, Horiba Jobin Yvon, France) was used with 532 nm Ar-laser beam and scan range from 400 cm−1 to 2200 cm−1.3. Results and discussion
3.1 Selecting of effective co-milling reagents
It has been proved that PS can be activated by Fe2+ or alkali, so we investigated the effectiveness of FeSO4·7H2O and NaOH as co-milling reagents in company with PS. In order to get an appropriate mass ratio, 4.17 g of PS was adopted in the case of PS + activator, considering both the degradation efficiency and the cost. F-53B was co-ground with PS, NaOH or the mixture of PS and different activators (FeSO4·7H2O, NaOH) for 8 h. In the case of PS + activator, a series of mass ratios of PS/NaOH and PS/FeSO4·7H2O were studied, and the defluorination and destruction results obtained from the respective best mass ratios are shown in Fig. 1 (S1: PS 5.92 g; S2: NaOH 5.92 g; S3: PS 4.17 g, FeSO4·7H2O 0.2 g; S4: PS 4.17 g, NaOH 1.75 g). The defluorination and F-53B degradation efficiencies were extremely low when using PS, NaOH or PS–FeSO4·7H2O. The fluoride recovery was highest when using NaOH as activator and the F-53B destruction efficiency was quite high, which indicates the effective destruction of the F-53B has been realized. Therefore, it is suggested that the combination of PS and NaOH can be an effective co-milling reagent for the MC destruction of F-53B.3.2 Effect of PS/NaOH mass ratio
For the purpose of choosing the best ratio of PS and NaOH, a series of ball milling experiments were further conducted and the results are shown in Fig. 2. As we can see, the fluoride recovery increased with the increase of NaOH and reached the highest point when the dosage of NaOH was 1.75 g. However, when we further increased the dosage of NaOH, the fluoride recovery began to decrease. The F-53B remaining had a negative correlation with the fluoride recovery, and the best F-53B degradation point was consistent with the highest fluoride recovery point, which demonstrates defluorination is one of the most important way to degrade F-53B. It is universally known that base is the most commonly used activator of PS. Previous studies have revealed that there were SO4˙−, ˙OH and superoxide radical (O2˙−) in base-activated PS reactions, which play significant roles in the oxidization of the hazardous compounds.18,21,22 So we infer that when the mass of NaOH was low, there were not enough radicals, and with the increase of NaOH, more and more radicals were released and took part in the reaction, therefore, leading to more fluoride recovery and less F-53B remaining.| SO4˙− + SO4˙− → 2SO42−, k = 4.0 × 108 M−1 s−1 | (4) |
| ˙OH + ˙OH → H2O2, k = 5.5 × 109 M−1 s−1 | (5) |
| ˙OH + SO4˙− → HSO4− + 1/2O2, k = 1.0 × 1010 M−1 s−1 | (6) |
 | ||
| Fig. 2 Eight hours MC treatment of F-53B with different mass ratio of PS to NaOH (PS was kept constant at 4.17 g). | ||
When the mass of NaOH continued to increase, the release rate of radicals would be accelerated, causing more radicals generated in a short time, as indicates by eqn (3). However, the high concentration of the radicals may give rise to various radical scavenging reactions (eqn (4)–(6)). Consequently, less radicals took part in the reaction with the pollutant, as a result, the F-53B degradation and defluorination efficiencies decreased.22,23 Besides, when the mass of NaOH continued to increase, the charge ratio (the ratio of the total mass of the balls to the total mass of the mixture) was decreased, and the impact energy was insufficient to induce the MC degradation.24 Researches showed that H2O can give rise to the agglomeration of the sample, which contributes to the decrease of the reaction speed,25 NaOH can absorb water from the air during the weighing process, the more NaOH, the more H2O (when 3.15 g NaOH was used, the mass of the reagent increased 0.01 g more than the case of 1.75 g NaOH). We studied the effect of the increase of H2O on the degradation efficiency of F-53B (adding 0.01 g H2O into the reagent of 1.75 g NaOH group) and found that the influence could be neglected (the defluorination and degradation efficiency with and without H2O were 52% and 85%, and 54% and 88%, respectively). This may be due to that the absorption of the H2O was too little.
The effect of the mass of PS on the degradation of F-53B was also studied, and the results were provided in Text S2 and Fig. S3.†
3.3 Effect of milling time
To ascertain the appropriate milling time for effective destruction of F-53B, a series of experiments were conducted, during which the best ratio (PS 4.17 g, NaOH 1.75 g) of PS and NaOH was used. The fluoride recovery and F-53B remaining were used to evaluate the degradation efficiency of F-53B.The results shown in Fig. 3 indicate that during the ball milling experiment, the F-53B remaining decreased gradually and the fluoride recovery was negatively correlated with the F-53B remaining. There was only 12% F-53B remaining after 8 h of milling and the fluoride recovery was 54% correspondingly. Moreover, there was a time interval between the F-53B remaining and the fluoride recovery, as shown in Fig. 3. The F-53B degradation was nearly 50% after 5 min of milling, while the fluoride recovery was only less than 4%. This phenomenon is in agreement with several previous studies,26,27 and Zhang et al. attributed such delay of dehalogenation to the formation of intermediates containing halogen.28
Most of F-53B was destructed during the first two hours and the F-53B remaining almost kept invariant in the next six hours. Zhang et al.25 reported that the generation of H2O during the ball milling can give rise to the agglomeration of the sample, which contributed to the decrease of the reaction speed. Actually, the reagents stuck together with the increase of milling time and the samples collected after two hours were obviously wet. The contact area between the balls and the reagents became smaller, causing the reactions difficult to happen.
While our studies demonstrated that the effective degradation of F-53B can be realized, to further evaluate the mineralization effect, TOC analysis was conducted. Consistent with the degradation of F-53B, the TOC contents of the samples decreased rapidly with the increase of milling time, and compared with the initial value, 43% of TOC left after 8 h of milling, which demonstrates mineralization is an important way to degrade F-53B, a majority of F-53B can be degraded completely and there was small part of intermediates left.
The decomposition of PS at different milling time was also measured (as shown in Fig. 4). During the first two hours, more than 90% PS was consumed; and less than 1% PS remained after 8 h of milling. The ether bond is more likely to be attacked by NaOH activated PS and leads to the decomposition of F-53B. Both PS and F-53B can release sulfate and the majority of sulfate (more than 99%) comes from PS. With increased milling time, sulfate recovery increased gradually (as shown in Fig. 4), which demonstrates the decomposition of PS.
3.4 Characterization of milled samples
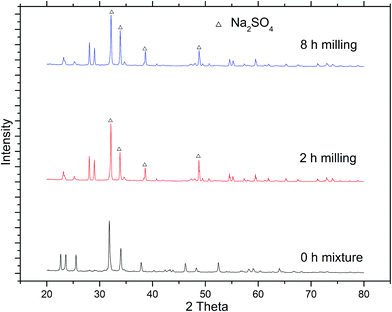
FTIR analysis was conducted to further confirm the degradation of F-53B, and the spectra are shown in Fig. 5. The peak around 1200–1350 cm−1 from the vibration of –CF3 and –CF2 groups can be used as a monitor of organic fluorine.29,30 In the spectra of 0 h mixture sample, the typical peak band was very strong, while for the 2 h and 8 h milled samples, the peak band disappeared. What's more, there were no typical peak bands for Na2SO4 in the spectrum of 0 h mixture, while for the samples milled for 2 h or 8 h, typical peak bands of Na2SO4 at 617![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 635 and 1124 cm−1 were clearly observed.
635 and 1124 cm−1 were clearly observed.
On the other hand, the fracture of C–F bonds was also proved by XPS analysis, and the F (1s) spectrum is shown in Fig. 6. The dominant peak at 689 eV is attributed to the C–F bonds and the peak at 684 eV is assigned to the negatively charged monovalent fluorines (F−).30 There was only a strong peak at 689 eV in the spectrum of pure F-53B, nevertheless, a new peak at 684 eV appeared after 2 h of milling. In the map of 8 h milled sample, the peak around 689 eV was weaker and the peak centered at 684 eV got stronger compared with the 2 h milled sample.
Fig. 7 shows the XRD patterns of the samples with different milling time. For the samples milled for 2 h or 8 h, there were apparent peaks of Na2SO4, which was consistent with the results of FTIR and ion chromatography analysis for SO42−, while the peaks of Na2SO4 were not observed in the map of 0 h mixture.
When the milled sample was dissolved with ultrapure water, there was no solid remaining, however, the milled sample can hardly be dissolved in methanol, and the separated solid residue was characterized (Text S3†). The results shown in Fig. S4 and S5† implied the majority of the residue was Na2SO4 and no F-53B remained.
3.5 Mechanism of the reaction
Although no peaks representing graphite or amorphous carbon were found in the Raman spectra (data not shown) of the milled samples, the mineralization was confirmed to occur. We infer that the carbon may be released in the form of carbon dioxide or carbon oxide in view of the generation of gas during the milling process.12,31 PS-assisted MCD process for the degradation of F-53B involved the breakage of C–F and C–C bonds and the generation of inorganic fluoride. The peak of C–F bond disappeared in the FTIR spectrum of 2 h milled sample, while the fluoride recovery was only 40%, which makes us consider that fluorine may exist in some unique forms (besides ionic and organic forms). The generation of gas during the milling process needs further study.According to the discussions above, the degradation pathways of F-53B in the PS-assisted MCD process were proposed as follows: the sulfonate are easy to be attacked, resulting in the breakage of C–S bond with the generation of sodium sulfate and organic intermediates; then the fluorinated intermediates react with a series of nucleophilic groups and the fluorides at the end of the carbon chain being replaced,30 consequently the carbon chain become shorter and shorter until completely mineralized. During the process, the organic carbons convert to carbon dioxide or carbonate and the fluorines change to ionic or other forms.
4. Conclusions
The mixture of PS–NaOH can be used to destruct F-53B in the mechanochemical process, and the best results (88% F-53B degradation and 54% fluoride recovery) can be achieved at the optimal ratio (PS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH
NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F-53B = 4.17
F-53B = 4.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.75
1.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05) after 8 h of milling. When the amount of PS is enough, the degradation efficiency of F-53B is closely related to the dosage of NaOH. TOC analysis confirmed the mineralization of F-53B, while FTIR and XPS demonstrated the cleavage of C–F bonds. Defluorination and mineralization were proposed as the main pathways of F-53B destruction.
0.05) after 8 h of milling. When the amount of PS is enough, the degradation efficiency of F-53B is closely related to the dosage of NaOH. TOC analysis confirmed the mineralization of F-53B, while FTIR and XPS demonstrated the cleavage of C–F bonds. Defluorination and mineralization were proposed as the main pathways of F-53B destruction.
Acknowledgements
The study was supported by the Ministry of Science and Technology (Project No. 2013AA06A305), the National Natural Science Foundation (Project No. 21547001) and the Ministry of Environmental Protection of China (Project No. 201309044).References
- K. Prevedouros, I. T. Cousins, R. C. Buck and S. H. Korzeniowski, Environ. Sci. Technol., 2006, 40, 32–44 CrossRef CAS.
- A. G. Paul, K. C. Jones and A. J. Sweetman, Environ. Sci. Technol., 2009, 43, 386–392 CrossRef CAS.
- J. A. Bjorklund, K. Thuresson and C. A. De Wit, Environ. Sci. Technol., 2009, 43, 2276–2281 CrossRef.
- S. Wang, J. Huang, Y. Yang, Y. Hui, Y. Ge, T. Larssen, G. Yu, S. Deng, B. Wang and C. Harman, Environ. Sci. Technol., 2013, 47, 10163–10170 CAS.
- T. Ruan, Y. Lin, W. Thanh, R. Liu and G. Jiang, Environ. Sci. Technol., 2015, 49, 6519–6527 CrossRef CAS PubMed.
- H. Hori, A. Yamamoto and S. Kutsuna, Environ. Sci. Technol., 2005, 39, 7692–7697 CrossRef CAS.
- H. Moriwaki, Y. Takagi, M. Tanaka, K. Tsuruho, K. Okitsu and Y. Maeda, Environ. Sci. Technol., 2005, 39, 3388–3392 CrossRef CAS.
- Q. Zhuo, S. Deng, B. Yang, J. Huang and G. Yu, Environ. Sci. Technol., 2011, 45, 2973–2979 CrossRef CAS PubMed.
- C. D. Vecitis, H. Park, J. Cheng, B. T. Mader and M. R. Hoffmann, Front. Environ. Sci. Eng. China, 2009, 3, 129–151 CrossRef CAS.
- H. Duan, J. Li, Y. Liu, N. Yamazaki and W. Jiang, Environ. Sci. Technol., 2011, 45, 6322–6328 CrossRef CAS PubMed.
- K. Zhang, J. Huang, G. Yu, Q. Zhang, S. Deng and B. Wang, Environ. Sci. Technol., 2013, 47, 6471–6477 CAS.
- K. Zhang, J. Huang, H. Wang, G. Yu, B. Wang, S. Deng, J. Kano and Q. Zhang, RSC Adv., 2014, 4, 14719–14724 RSC.
- S. A. Rowlands, A. K. Hall, P. G. McCormick, R. Street, R. J. Hart, G. F. Ebell and P. Donecker, Nature, 1994, 367, 223 CrossRef PubMed.
- K. Zhang, J. Huang, W. Zhang, Y. Yu, S. Deng and G. Yu, J. Hazard. Mater., 2012, 243, 278–285 CrossRef CAS PubMed.
- Y. Yu, J. Huang, W. Zhang, K. Zhang, S. Deng and G. Yu, Chemosphere, 2013, 90, 1729–1735 CrossRef CAS PubMed.
- H. Wang, J. Huang, K. Zhang, Y. Yu, K. Liu, G. Yu, S. Deng and B. Wang, J. Hazard. Mater., 2014, 264, 230–235 CrossRef CAS PubMed.
- C. Qi, X. Liu, W. Zhao, C. Lin, J. Ma, W. Shi, Q. Sun and H. Xiao, Environ. Sci. Pollut. Res. Int., 2015, 22, 4670–4679 CrossRef CAS PubMed.
- O. S. Furman, A. L. Teel and R. J. Watts, Environ. Sci. Technol., 2010, 44, 6423–6428 CrossRef CAS PubMed.
- C. Qi, X. Liu, C. Lin, X. Zhang, J. Ma, H. Tan and W. Ye, Chem. Eng. J., 2014, 249, 6–14 CrossRef CAS PubMed.
- H. Lee, H.-J. Lee, J. Jeong, J. Lee, N.-B. Park and C. Lee, Chem. Eng. J., 2015, 266, 28–33 CrossRef CAS PubMed.
- D. Zhao, X. Liao, X. Yan, S. G. Huling, T. Chai and H. Tao, J. Hazard. Mater., 2013, 254, 228–235 CrossRef PubMed.
- C. Liang, Y. Y. Guo and Y. R. Pan, Int. J. Environ. Sci. Technol., 2014, 11, 483–492 CrossRef CAS.
- Y. Ji, C. Ferronato, A. Salvador, X. Yang and J.-M. Chovelon, Sci. Total Environ., 2014, 472, 800–808 CrossRef CAS PubMed.
- H. Wang, J. Huang, K. Zhang, Y. Yu, K. Liu, G. Yu, S. Deng and B. Wang, J. Hazard. Mater., 2014, 264, 230–235 CrossRef CAS PubMed.
- Q. W. Zhang, J. F. Lu, F. Saito and M. Baron, J. Appl. Polym. Sci., 2001, 81, 2249–2252 CrossRef CAS PubMed.
- A. K. Hall, J. M. Harrowfield, R. J. Hart and P. G. McCormick, Environ. Sci. Technol., 1996, 30, 3401–3407 CrossRef CAS.
- W. Zhang, J. Huang, F. Xu, S. Deng, W. Zhu and G. Yu, J. Hazard. Mater., 2011, 198, 275–281 CrossRef CAS PubMed.
- K. Zhang, J. Huang, W. Zhang, Y. Yu, S. Deng and G. Yu, J. Hazard. Mater., 2012, 243, 278–285 CrossRef CAS PubMed.
- Y. H. Kim, M. S. Hwang, H. J. Kim, J. Y. Kim and Y. Lee, J. Appl. Phys., 2001, 90, 3367–3370 CrossRef CAS PubMed.
- K. Zhang, J. Huang, G. Yu, Q. Zhang, S. Deng and B. Wang, Environ. Sci. Technol., 2013, 47, 6471–6477 CAS.
- Q. W. Zhang, H. Matsumoto and F. Saito, Chem. Lett., 2001, 148, 148–149 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15337a |
| This journal is © The Royal Society of Chemistry 2015 |