Fabrication of MEA based on sulfonic acid functionalized carbon supported platinum nanoparticles for oxygen reduction reaction in PEMFCs†
Hussein Gharibi*ab,
Fatemeh Yasia,
Mohammad Kazemeinic,
Ahmad Heydaria and
Farhad Golmohammadia
aDepartment of Chemistry, Faculty of Science, Tarbiat Modares University, P.O. Box 14115-175, Tehran, Iran. E-mail: h.gharibi@utah.edu; h.gharibi@gmail.com; gharibi@modares.ac.ir
bDepartment of Material Science & Engineering, 122 S Campus Drive, University of Utah, Salt Lake City, UT 84112, USA
cDepartment of Chemical and Petroleum Engineering, Sharif University of Technology, Tehran, Iran
First published on 24th September 2015
Abstract
The Nafion ionomer affects the efficiency of the platinum (Pt) catalyst by blocking the active sites thereby restricting the gas permeability of the catalyst layer; but, there is a limitation in the quantity of Nafion ionomer that needs to be added without affecting the cell performance. Sulfonation of carbon-supported catalysts as mixed electronic and protonic conductors has been reported to be an efficient way to increase the triple-phase boundaries. In order to improve the utilization and activity of cathodic catalysts in the oxygen reduction reaction (ORR), Pt nanoparticles were loaded on a mixture of Vulcan XC-72R and MWCNTs, which were functionalized in a mixture of 96% sulfuric acid and 4-aminobenzenesulfonic acid using sodium nitrite to produce intermediate diazonium salts from substituted anilines. The influence of sulfonation on the structural, surface, morphological and catalytic characteristics of the catalysts was explored using X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy and electrochemical techniques. The performance of the ORR was optimal under the following conditions: 75 wt% f-MWCNTs and 25 wt% f-Vulcan XC-72R (5%). The optimum loading of Nafion was found to be 15 wt% and the MEA was fabricated according to this Nafion loading which is lower than that of other MEAs. The maximum power density of MEA with the modified electrocatalyst was 1.6 times more than that of MEA with the unmodified electrocatalyst.
1. Introduction
Polymer electrolyte membrane fuel cells (PEMFCs) are attractive alternative power sources due to their safe emissions, high conversion efficiency, and high energy density.1,2 The objectives necessary for technical application and commercialization of PEMFCs can be classified as two aspects: increasing the activity of the oxygen reduction reaction (ORR) and increasing the catalyst stability.3–5 For PEMFCs, electrode performance relies critically on the three phase boundary formations in the membrane-electrode-assembly (MEA).6 A high performance electrode can provide effective proton conduction, electron conduction, and a transfer path for reactant and product to or from the catalyst layer. Hydrophobic polytetrafluoroethylene (PTFE) is added into the gas diffusion layer to enhance water management, whereas Nafion ionomer is mixed with the catalyst later (CL) to improve the utilization of Pt catalyst. The Nafion ionomer fails to penetrate the small pores in the carbon agglomerate. Besides, Nafion ionomer often affects the efficiency of the Pt catalyst by blocking the active sites thereby restricting the gas permeability of the catalyst layer as well as its electronic conductivity. Accordingly, there is a limit to the quantity of Nafion ionomer that needs to be added without affecting the cell performance.7 Several studies have focused on the enhancement of catalyst utilization in PEFC electrodes by extending the so-called triple-phase boundaries.8,9 Among these, sulfonation of carbon-supported catalysts as mixed electronic and protonic conductors has been reported to be an efficient way to increase the triple-phase boundaries. Electrochemical characterization has shown that the catalysts supported on functionalized carbons had a better performance for alcohol electro-oxidation,10–13 formic acid electro-oxidation,14,15 EG electro-oxidation16 and oxygen reduction reaction7,8,17–24 as compared to unsulfonated counterparts. Electrodes modified with functional sulfonic acid groups exhibited better performance compared to conventional electrodes with unsulfonated counterparts mainly due to the easier access to protons and well dispersed distribution of the sulfonated catalysts. Sulfonic acid groups were grafted chemically onto the surface of carbon particles. This procedure aimed for a better interaction of the proton-conducting phase with the metallic catalyst and so increased the triple phase boundary zone of the catalysts.Herein, phenyl-sulfonic acid groups were grafted onto the catalyst carbon surfaces, as promising supports for the Pt catalysts. Much research has focused on the most commonly used carbon black supports, e.g. Vulcan XC-72R. Recently CNTs, as innovative catalyst supports, have drawn a great deal of attention due to their unique surface structures, excellent mechanical and thermal properties, high electric conductivity, and large surface areas.22 Compared with the binary support electrode, the current on the single-CNT support electrode experienced little change at low overpotentials, but deteriorated rapidly at high overpotentials. The difference in the polarization features might be associated with the wettability and structural properties of the carbon support materials used. As CNTs have a hollow tubular structure and the CNT support electrode has many micro or nanosized pores, the catalyst layer was easily penetrated by the electrolyte solution under the action of capillarity. As a result of this action, a decreased interfacial area of the gas–liquid–solid phase at the reaction sites and larger flooding may occur for the single-CNT support electrode.25 In this work, sulfonation extended from the Pt/C and Pt/MWCNT catalysts to the mixture of Vulcan XC-72R and MWCNT supported Pt catalysts. The well dispersed reduction of Pt nanoparticles on the surface of benzenesulfonic functionalized MWCNTs and Vulcan XC-72R was demonstrated with a H2PtCl6 aqueous solution by using NaBH4 as a reductive agent. The Pt catalysts supported on sulfonated MWCNTs were characterized in detail by scanning electron microscopy (SEM), Fourier transform infrared (FTIR), X-ray diffraction (XRD), cyclic voltammetry, electrochemical impedance spectroscopy (EIS) and chronoamperogram techniques. The modified catalyst exhibited a high catalytic activity than traditional Pt/C and Pt/MWCNT catalysts. Single cell evaluation of the as-prepared catalyst showed significant improvement in the oxygen reduction reaction performance. Finally, this is the first attempt to utilize this modified Pt/C cathode in a proton exchange membrane fuel cell. Also, we showed that this MEA exhibited better performance compared to the MEA prepared from Pt/C (Electrochem), as characterized by polarization curves and impedance diagrams.
2. Instrument and measurement
X-ray diffraction (XRD) analysis was carried out for the catalysts by using an X'PERT MPD Philips diffractometer with a Co X-ray source operating at 40 kV and 40 mA. The XRD patterns were obtained at a scanning rate of 1° min−1 with a step size in the 2θ scan of 0.02° in the range 10–90°. A transmission electron microscope (TEM) operated at 200 kV (Philips Model CM200) was applied for analysis of the morphology of electrocatalysts. Infrared spectra were recorded with a model IR 100 Nicolet FTIR spectrophotometer. The electrochemical measurements were performed using the EG&G Princeton Applied Research Model 2273 instrument. All experiments were performed at room temperature in a conventional three-electrode cell assembled with a glassy carbon (GC) disk as the working electrode (1 mm in diameter), Ag/AgCl as a reference and Pt foil as the counter electrodes. The amount of loading of catalyst was 0.5 mgmetal cm−2. The reduction of oxygen was investigated with porous GDE (geometric exposed area of 1.3 cm2) in a 0.5 M H2SO4 solution. The GDEs were mounted on a Teflon holder, holder containing a high pyrolytic graphite disk as a current collector (with an oxygen feed from the back of the electrode). A large-area platinum flat electrode was used as the counter electrode. An Ag/AgCl reference electrode was placed close to the surface of the working electrode. The electrochemical cell was connected to a potentiostat–galvanostat (EG&G Model 2273) for I–V polarization measurements (LSV), chronoamperometry (CA), and electrochemical impedance spectroscopy (EIS).A fuel cell test station (FCT-PaxiTech) was used to measure cell polarization and impedance. Hydrogen and oxygen were passed through temperature-controlled humidifiers to reflect on the separate humidifiers used for oxygen and hydrogen. Each MEA was initially activated at 0.6 V for 2 hours at 100% relative humidity (RH); the voltage scan measurements were performed between 1 and 0.05 V at a scan rate of 10 mV s−1.26 The humidification temperatures of the anode and cathode gases and cell temperature were varied. The flow rates were 300 mL min−1 for hydrogen and 500 mL min−1 for O2. The gas pressure at the back of the electrodes was 1.5 atm.
3. Experimental
3.1. Anchoring phenyl-sulfonic groups to Vulcan XC-72R using p-amino phenyl-sulfonic acid
The process of preparation of benzenesulfonic functionalized Vulcan XC-72R has been reported elsewhere.5 In a typical experiment of synthesis of f-Vulcan XC-72R, carbon support was mixed with a hot aqueous solution of p-amino phenyl sulfonic acid (Merck, Germany) to form a slurry. The required amount of concentrated hydrochloric acid was added drop wise to the carbon slurry with constant stirring at 80 °C. The resulting suspension was cooled below 20 °C. Subsequently, the aqueous solution of sodium nitrite was stirred in to form 4-sulfobenzenediazonim salts in situ that reacted with the carbon support (Fig. S1†). The mixture was stirred for 30 min, filtered, washed copiously with hot distilled water to remove the unbounded residues, and then dried in an air oven at 120 °C for 2 h.3.2. Purification of raw MWCNTs (p-MWCNTs)
The MWCNTs were refluxed with concentrated nitric acid at 120 °C for 12 h, then washed and filtered with deionized water. The steps introduced oxygen containing groups, mainly carboxyl groups to the MWCNTs.27 We denoted them as p-MWCNT in the following text. The solid phase was filtered and then dried in air at 80 °C for 12 h.133.3. Functionalization of MWCNTs (f-MWCNTs)
The procedures of benzenesulfonic functionalized MWCNTs have been described elsewhere.14 A typical experiment consisted of dispersing p-MWCNT in 96% H2SO4 and (NH4)2S2O8 by magnetic stirring (6 h). When the mixture was visibly dispersed, the substituted 4-aminobenzenesulfonic acid was added and homogenization was continued for 2 h in order to effectively disperse the aniline throughout the mixture. This was followed by the addition of solid NaNO2 and a slow addition of di-tert-butyl peroxide (AIBN and di-tert-butyl peroxide produced similar results). The mixture was stirred at 80 °C and homogenization was continued for 6 h. The resulting product was filtered and washed with deionized water, acetone, and fresh N,N-dimethylformamide (DMF) in order to remove any impurity and then dried at 30 °C for 24 h in a vacuum oven.3.4. Preparation of carbon-supported platinum catalyst
The modified catalysts with different ratios of f-MWCNTs and f-VulcanXC-72R were synthesized at 80 °C by using NaBH4 as a reductive agent. The loading of the phenyl sulfonic acid group anchored to Vulcan XC-72R was 5 w/o. The mixture of f-VulcanXC-72R and f-MWCNTs were impregnated with Pt particles by adding H2PtCl6·6H2O salt in water followed by sonication of the suspension for 30 min. Excess quantities of 0.1 M NaBH4 solution were added drop wise to the suspension with vigorous stirring at 80 °C. The mixture was stirred for 24 h at 80 °C to permit the complete reduction of Pt from its metal salt. Finally, the resulting material was washed with distilled water several times and dried at 70 °C. The metal loading was determined by ICP to be 10 wt%.3.5. Gas-diffusion layer (GDL) fabrication
The diffusion layer was prepared from a suspension consisting of carbon and PTFE (6% solution by weight) in distilled water and sonicating the mixture for 30 minutes at room temperature. The amount of PTFE in the diffusion layer was 30% (by weight), and the loading in the carbon and PTFE composite was 1 mg cm−2.28 The suspension was painted onto the porous carbon paper and then dried at 80 °C for 30 min. The electrodes were heated at 280 °C for 30 min to remove the dispersing agent (from the PTFE) and then sintered at 330 °C for 30 min.3.6. Preparation of electrode
For the electrode preparation, typically, the desired amount of electrocatalyst was added into the Nafion solution (5% from Aldrich), 2-propanol (Merck) and water, and then the mixture was treated 20 min with ultrasonication for uniform dispersion. This suspension was painted onto the diffusion layer. The electrode was dried at 80 °C for 2 h. The Pt loading was 0.5 mg cm−2. The amount of f-MWCNT in GDEs was varied according to Table 1. These electrodes were used in I–V polarization measurements, chronoamperometry, and electrochemical impedance spectroscopy. For the cyclic voltammetry measurement, glassy carbon (GC) working electrodes 1 mm in diameter (electrode area: 0.031415 cm2) were used as substrates for the catalysts. For the electrode preparation, a measured volume of the prepared ink was dropped by a micropipette onto the top surface of the GC electrode. The obtained catalysts modified GC electrode was employed as the working electrode in our experiments.| GDE | % f-Vulcan XC-72R content | w/o of phenyl sulfonic acid anchored on to f-Vulcan XC-72R | % f-MWCNT content | % Nafion content |
|---|---|---|---|---|
| 10 | 100 | 0 | 0 | 15 |
| 20 | 100 | 5 | 0 | 15 |
| 30 | 0 | 0 | 100 | 15 |
| 11 | 75 | 5 | 25 | 15 |
| 12 | 50 | 5 | 50 | 15 |
| 13 | 25 | 5 | 75 | 15 |
4. Results and discussion
4.1. Physical characterization of catalysts
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH, H–OH (adsorbed molecular water) may be created at the surface. The presence of these polar groups may lead to form the homogeneous solution in water, which leads to the facile loading of nanoparticles on the substrate (10% wt Pt).
O, C–OH, H–OH (adsorbed molecular water) may be created at the surface. The presence of these polar groups may lead to form the homogeneous solution in water, which leads to the facile loading of nanoparticles on the substrate (10% wt Pt).A certain amount of modified supports was stirred in the excess amount of 6 × 10−3 N NaOH aqueous solution for 24 h. The mixture was then filtered and the exact amount of the filtrate was titrated with 6 × 10−3 N HCl aqueous solution. The amount of acid groups in the modified supports was estimated by the NaOH consumed.29 The results are shown in Table 2.
| Support | Acid densitya (amount of functional group (g) × 100/weight of support (g)) | wt% of surface S as –SO3H (EDS) | Acid densityb (amount of functional group (g) × 100/weight of support (g)) |
|---|---|---|---|
| a Acid–base back titration. Catalyst reacted with excess 6 × 10−3 N NaOH aqueous solution for 24 h before neutralizing.b Based on S elemental analysis. | |||
| f-Vulcan XC-72 5% | 5.90 | 1.13 | 5.53 |
| f-MWCNT | 11.06 | 2.19 | 10.76 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and –OH group present on the carbon support, respectively.
C and –OH group present on the carbon support, respectively.The modified catalyst shows a broad intense-band in the region 1300–3300 cm−1, which is attributed to the –OH group attached to sulfur in the sulfonic acid group. The strong bands around 1700 cm−1, 1090 cm−1 and a weak band near 1385 cm−1 are attributed to the stretching mode of the sulfite group. These observations confirm that the phenyl-sulfonic acid groups are anchored to the carbon support.7
The possibility of functionalized MWCNTs with benzenesulfonic group was examined by IR analysis of f-MWCNTs to confirm such interaction. The result is shown in Fig. S2.† The peaks at 729, 1630, 2916 and 2971 cm−1 were attributed to adding the –CH2– group of the aromatic ring system. The peaks at 681 and 1170 cm−1 can be assigned to the S–O group of the sulfonate group.7
| GDE | QH (mC cm−2) | ECSA (m2 gPt) | Average particle size (nm) | Tafel slope (mV dec−1) | Exchange current density (A cm−2) |
|---|---|---|---|---|---|
| 10 | 37.63 | 35.84 | 5.24 | 110.55 | 4.47 × 10−6 |
| 20 | 63.52 | 60.30 | 4.26 | 88.17 | 1.75 × 10−5 |
| 30 | 64.76 | 61.68 | 3.87 | 87.84 | 1.78 × 10−5 |
| 11 | 68.49 | 65.13 | 3.63 | 86.70 | 2.58 × 10−5 |
| 12 | 70.73 | 67.36 | 3.42 | 86.43 | 2.66 × 10−5 |
| 13 | 113.36 | 126.05 | 2.10 | 77.65 | 3.41 × 10−5 |
4.2. Electrochemical measurements
To study the effects of synergism on the ORR performance of the GDEs, GDEs were prepared from mixtures of f-MWCNTs and f-Vulcan XC-72R as carbon substrates with various physical properties. An EG&G Princeton Applied Research Model 2273 was used in the electrochemical measurements. The reduction of oxygen at each porous GDE (geometric exposed area 1 cm2) was investigated in 0.5 M H2SO4 as the electrolyte. All measurements were performed at 25 °C in a conventional three-electrode cell with flowing oxygen.
 | (1) |
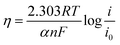 | (2) |
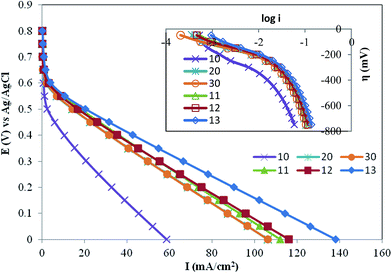 | ||
| Fig. 2 I–V curves at 25 °C for different catalysts in a 0.5 mol L−1 H2SO4 solution at a scan rate of 1 mV s−1 (Pt loading: 0.5 mg cm−2). | ||
The kinetics data for different GDEs were obtained in the LCD region potential range and listed in Table 3. Excellent linear correlations are found between 0.4508 and 0.60 V in each case, and Table 3 collects the corresponding Tafel slopes. In the Tafel region and the mixed potential region, we compared the results for remarkable differences between their maximum current densities in the high potential region and found that the catalytic activity of GDE 11 (75 wt% of functionalized Vulcan XC-72 in the catalyst support), GDE 12 (50 wt% of f functionalized Vulcan XC-72 in the catalyst support) and GDE 13 (25 wt% of functionalized Vulcan XC-72 in the catalyst support) toward the ORR was higher than that of GDEs 10, 20 and 30 (which contain pure supports in the catalyst layer) with respect to the ECSA normalized with an electrode real surface area. This is probably due to the extension of the three-phase boundary in the catalyst layer, especially inside the carbon agglomerate through phenyl-sulfonic acid group.7 The highest catalytic activity for the ORR among all the electrocatalysts was found with GDE 13. Based on the ECSA of Pt, however, all the modified electrocatalysts show a surface active area enhancement in comparison with the unmodified electrocatalyst. The loading of a functionalized group anchored on Vulcan XC-72R was 5 w/o. However, further increase in phenyl sulfonic acid loading decreased efficiency possibly due to the increased hydrophilic nature of the catalyst layer.
The potential (E) versus current density (i) data were analyzed fitting the data to equation:34
E = Er + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0 − b i0 − b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i − iR i − iR
| (3) |
 | (4) |
At high frequencies, a Warburg-like response (45° slope) was observed, corresponding to ion migration through the catalyst layer. The ionic resistance Rion can be obtained from the length of the Warburg-like region projected onto the real impedance (Zreal) axis.37 To obtain Rion, the impedance measurements were used to create open-circuit voltage (OCV) potential. Nyquist plots of the impedance response from 100 kHz to 50 mHz (OCV at 25 °C) in saturated argon were obtained. The ionic resistance (Rion) could be satisfactorily derived from the impedance data through application of the following equation:38
 | (5) |
| Catalyst | Rct (Ω) | Rion/3 | i (mA cm2) | ||
|---|---|---|---|---|---|
| 0.3 (V vs. Ag/AgCl) | 0.6 (V vs. Ag/AgCl) | 0.3 V | 0.6 V | ||
| GDE 10 | 3.38 | 4.00 | 28.84 | 20.82 | 0.82 |
| GDE 20 | 1.65 | 3.43 | 20.04 | 49.94 | 1.94 |
| GDE 30 | 1.59 | 3.33 | 18.23 | 49.81 | 2.13 |
| GDE 11 | 1.29 | 2.63 | 13.85 | 54.94 | 3.13 |
| GDE 12 | 1.17 | 2.58 | 13.37 | 55.27 | 3.8 |
| GDE 13 | 0.84 | 1.84 | 10.51 | 66.39 | 4.11 |
4.3. Fabrication of membrane electrode assembly
Nafion-115 (DuPont) used as the proton electrolyte membrane, was treated in 30 w/o H2O2 for 1.5 h followed by rinsing and washing in distilled water. It was then boiled in 0.5 M H2SO4 for 1.5 h followed by washing with distilled water.Both anode and cathode comprise a gas diffusion layer and a reaction layer. To prepare the gas-diffusion layer, Vulcan-XC-72R carbon and PTFE (6% solution by weight) were suspended in isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water (2
distilled water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the mixture was sonicated for 30 min at room temperature. The amount of PTFE in the diffusion layer was 30% (by weight), and the loading in the carbon and PTFE composite was 1 mg cm−2. The resultant slurry was spread on a carbon paper and sintered in a furnace at 350 °C for 30 min. To prepare the reaction layer, the required amount of the catalyst (modified or unmodified) and Nafion (Dupont) solution were agitated for 1 h. The resulting ink was spread on the treated 115-Nafion membranes. The MEAs were assembled with gas diffusion electrodes and placed in a 6.25 cm2 Paxi-tech single-cell fuel cell for electrochemical testing. Both anode and cathode contain platinum loading of 0.5 mg cm−2 and Nafion loading of 0.9 mg cm−2.
1) and the mixture was sonicated for 30 min at room temperature. The amount of PTFE in the diffusion layer was 30% (by weight), and the loading in the carbon and PTFE composite was 1 mg cm−2. The resultant slurry was spread on a carbon paper and sintered in a furnace at 350 °C for 30 min. To prepare the reaction layer, the required amount of the catalyst (modified or unmodified) and Nafion (Dupont) solution were agitated for 1 h. The resulting ink was spread on the treated 115-Nafion membranes. The MEAs were assembled with gas diffusion electrodes and placed in a 6.25 cm2 Paxi-tech single-cell fuel cell for electrochemical testing. Both anode and cathode contain platinum loading of 0.5 mg cm−2 and Nafion loading of 0.9 mg cm−2.
4.3.1.1. Temperature effect. Temperature is one of the most important operating parameters that needs to be properly controlled. The major effect of temperature is to change the reaction kinetics of the ORR in the fuel cell; the other effect is to change the fuel cell's water management. Temperature can also affect proton transport inside the membrane, resulting in membrane conductivity change.
The following membrane electrode assembly (MEA) samples were tested in this experiment. MEA-1: 10 wt% Pt on 75% f-MWCNT + 25% f-Vulcan XC-72R used as cathode and 10 wt% commercial Pt/C used as anodes, MEA-2: 10 wt% commercial Pt/C used as cathode and anodes. MEA catalyst loadings for both the anode and cathode were 0.5 mgmetal cm−2. Fig. 4(a) shows the polarization and power density curves of the MEAs: 1 and 2 under identical testing conditions. The MEA's were initially activated at 0.6 V for 2 h at 100% relative humidity (RH); the voltage scan measurements were performed between 0.05 and 1.2 V at a scan rate of 50 mV s−1.39 The humidification temperatures of the anode and cathode gases were varied; the flow rates were 300 mL min−1 for hydrogen and 500 mL min−1 for O2.
According to the polarization curves obtained at 50, 60, 70 and 80 °C the best performance of MEA-1 was obtained at 80 °C. In the high current density range, all performances dropped rapidly, which was typical of mass transfer limitation. However, an increase in temperature makes a huge difference in the high current density range, due to improvements in mass transfer at higher temperatures. Furthermore, MEA-1 shows comparable activity to MEA-2 in the high current density region. The better performance of MEA-1 confirms the above obtained parameters (conventional three electrode system) and suggests that modified electrocatalyst as cathode high catalytic activity for oxygen reduction in a proton exchange membrane fuel cell. Table 5 shows the kinetic parameters obtained for the MEA-1 from polarization curves. As shown in Table 5, the kinetic parameters of the MEA-1 improved considerably in comparison with MEA-2 (Pt/C Electrochem 10%). This indicates that the current density improved in the presence of a functional group in the support. The temperature effect on oxygen limiting current results from its positive effect on oxygen diffusion and its negative effect on oxygen solubility in the Nafion ionomer.40
| MEA | Cell temperature (°C) | Back pressure (atm) | RH (%) | i0 (A cm−2) | i0.3 (mA cm−2) | i0.6 (mA cm−2) | Rion/3 |
|---|---|---|---|---|---|---|---|
| MEA-1 | 80 | 1.5 | 60 | 3.28 × 10−3 | 805.51 | 360.54 | 0.0917 |
| 80 | 1.5 | 80 | 4.64 × 10−3 | 1421.6 | 625.28 | 0.0674 | |
| 80 | 1.5 | 90 | 7.30 × 10−3 | 1263.8 | 842.23 | 0.0164 | |
| 80 | 1.5 | 100 | 5.68 × 10−3 | 1110.7 | 666.62 | 0.0557 | |
| MEA-2 (Pt/C 10% Electrochem) | 80 | 1.5 | 90 | 3.60 × 10−3 | 819.05 | 518.98 | — |
As was mentioned, increasing the temperature will speed up the ORR. This is reflected in EIS in such a way that the kinetic arc in a Nyquist plot is larger at low temperatures than at high temperatures, as shown in Fig. S8.† However, the high frequency intercept value (membrane resistance) of the kinetic loop can be significantly affected by changing fuel cell temperature. Increasing temperature can reduce membrane resistance thereby improving fuel cell performance. As shown in Fig. S8,† at lower temperatures the arc radius in a low frequency range (mass transfer arc) can be significantly reduced by increasing fuel cell temperature as more liquid water may accumulate at the cathode, resulting in a mass transfer problem.
4.3.1.2. Humidity effect. Water management is an important issue in the performance of PEM fuel cells. Water content at the interface contributes to the transport of the involved species in many different ways. At one extreme, a high water level can block oxygen transport. If water content is elevated due to its generation at the cathode it can directly affect the ORR kinetics and also contribute indirectly to the state of contact between the Pt catalyst and the ionomer. If there is not enough water at the reaction interface the ionomer will shrink reducing both the surface contact of the catalyst with the ionomer and the proton conductivity of the ionomer. With respect to this, the amount of humidity in the reactant stream may affect the cell performance. Fig. 4(b) shows the polarization curves of a single cell at 80 °C and RHs of 60, 70, 80, 90 and 100%. The cell performance was strongly enhanced at higher relative humidity. For applications where a higher efficiency is required, a higher nominal cell potential may be selected, that is a RH of 80%. However, for applications where fuel cell size is important, a lower nominal cell potential may be selected, that is a RH of 90%, which would result in a higher power density. At higher relative humidity the cell performance decreased due to the increased hydrophilic nature of the catalyst in the presence of the phenyl-sulfonic acid group in the support, which led to flooding. The plot of η (overpotential) vs. log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i is shown in Fig. S9.† The exchange current density and the current densities at 0.3 V and 0.5 V of MEA-1 at different conditions are shown in Table 5, and are in agreement with charge transfer resistance from Fig. S10.†
i is shown in Fig. S9.† The exchange current density and the current densities at 0.3 V and 0.5 V of MEA-1 at different conditions are shown in Table 5, and are in agreement with charge transfer resistance from Fig. S10.†It is widely reported that humidity can influence the high-frequency intercept at the real axis, which is associated with membrane resistance. The conductivity of the membrane in a PEM fuel cell is directly related to its water content, which depends on: (1) the water carried by the humidified reactant gases; (2) the water generated by the cell reaction at the cathode; (3) the electroosmotic drag – that is, water carried by the protons that are transported from anode to cathode; and (4) back-diffusion of water from the cathode to the anode. As we know, the fuel cell EIS primarily represents the cathode behavior. Therefore, cathode humidification can greatly affect the whole impedance spectra. The humidification at the cathode causes a large difference in both the membrane resistance and the kinetic resistance. This result is reflected in the EIS Nyquist plot in Fig. S10.†
Fig. 5 shows impedance spectra obtained for MEAs at 0.3 and 0.5 V, which is related to the high current density (HCD) region; and at 0.7 V, which illustrates the behavior in the low current density (LCD) region. As shown in Fig. 5, the charge transfers kinetics in the LCD region (0.7 V) improved sharply in comparison with Pt/C Electrochem. The results are in consistency with the kinetic parameters obtained from the polarization curves.
However, impedance spectroscopy was employed to measure the proton conduction in MEA-1. AC impedance experiments under a N2 atmosphere were used to measure membrane resistance and the cathode proton resistance. Fig. 6 shows the impedance spectra obtained in OCV potential in different humidities. As shown in Fig. 6, reducing RH increases membrane resistance. The proton conduction resistance across the electrode can become rate determination and catalyst utilization limiting factor especially under low RH conditions and high current densities. The results of ionic resistance under different conditions are shown in Table 5.
4.3.1.3. The effects of temperature, pressure and relative humidity on the oxygen reduction reaction (ORR) by cyclic voltammetry, polarization and power density curves. The effects of temperature and relative humidity on the oxygen reduction reaction (ORR) in MEA-1 catalyst were investigated using cyclic voltammetry (CV) (Fig. S11†). CV studies were carried out to determine the electrochemical surface area and percentage of platinum utilization, which characterize the electrochemically active surface area of the electrode for hydrogen adsorption/desorption on platinum and its interface with Nafion. The electrochemical surface area and hydrogen adsorption charge from cyclic voltammetry diagrams are summarized in Table 6. As shown in Table 6, the electrochemical active surface area has not been significantly affected by temperature and relative humidity. This seems reasonable since the electrochemical active surface area of catalyst is a function of number of active sites of the surface which does not change significantly with temperature and relative humidity.
| Cell temperature | Absolute back pressure | Relative humidity | QH (C cm−2) | ECSA (m2 g−1 Pt) |
|---|---|---|---|---|
| 60 | 2.5 | 100 | 0.0392 | 37.37 |
| 70 | 2.5 | 100 | 0.0418 | 39.86 |
| 80 | 2.5 | 70 | 0.0576 | 54.89 |
| 80 | 2.5 | 80 | 0.0499 | 47.55 |
| 80 | 2.5 | 90 | 0.0798 | 76.05 |
| 80 | 2.5 | 100 | 0.0469 | 44.70 |
5. Conclusion
It was proposed that anchoring the phenyl-sulfonic group on the carbon support of the platinum catalyst would enhanced the performance of the PEMFC. The goal of this work was to investigate the synergism effect of the functionalized supports on the performance of PEMFCs. The combination of modified supports i.e.; 75% f-MWCNT + 25% f-Vulcan XC-72R (5%) was determined to be the optimum mixture. At this ratio, the electrochemical behavior of GDE13 was better than those electrodes containing more or less functional groups.According to the single cell data obtained, the synergism between the phenyl-sulfonic group and the Nafion ionomer affected the cell performance due to the increasing ion-conducting path in the catalyst layer and so enhanced catalyst utilization in the PEMFC electrodes by extending the so-called triple-phase boundaries. The related MEAs exhibited enhanced fuel-cell performance. The maximum power density of MEA with the modified electrocatalyst was 1.6 times more than that of MEA with the unmodified electrocatalyst.
However, further increase in phenyl sulfonic acid loading decreased Pt utilization possibly due to the increased hydrophilic nature of the catalyst layer, which leads to flooding albeit in the amelioration exhibited by negative charged platinum sites which helped repelling water molecules.
References
- K. Eom, Y. Y. Jo, E. Cho, T.-H. Lim, J. H. Jang, H.-J. Kim, B. K. Hong and J. H. Lee, J. Power Sources, 2012, 198, 42–50 CrossRef CAS PubMed.
- X. Yu and S. Ye, J. Power Sources, 2007, 172, 133–144 CrossRef CAS PubMed.
- S. Limpattayanate and M. Hunsom, J. Solid State Electrochem., 2013, 17(4), 1221–1231 CrossRef CAS.
- V. Mazumder, Y. Lee and S. Sun, Adv. Funct. Mater., 2010, 20, 1224–1231 CrossRef CAS PubMed.
- M. Sudan Saha, V. Neburchilov, D. Ghosh and J. Zhang, WIREs Energy and Environment, 2013, 2, 31–51 CrossRef PubMed.
- A. Therdthianwong, P. Saenwiset and S. Therdthianwong, Fuel, 2012, 91, 192–199 CrossRef CAS PubMed.
- G. Selvarani, A. K. Sahu, N. A. Choudhury, P. Sridhar, S. Pitchumani and A. K. Shukla, Electrochim. Acta, 2007, 52, 4871–4877 CrossRef CAS PubMed.
- R. H. Wu, K. S. Ho, T. E. Wei, T. H. Hsieh, Y. K. Han, C. W. Kuo, P. H. Tseng and Y. Z. Wang, Polymer, 2014, 55, 2035–2043 CrossRef CAS PubMed.
- S. Tominaka, K. Goto, T. Momma and T. Osaka, J. Power Sources, 2009, 192, 316–323 CrossRef CAS PubMed.
- C. Pak, S. H. Joo, D. J. You, J. M. Kim, H. Chang and D. Seung, Studies in Surface Science and Catalysis, ed. S. Q. Y. T. Dongyuan Zhao and Y. Chengzhong, Elsevier, 2007, vol. 165, pp. 401–404 Search PubMed.
- Z. P. Sun, X. G. Zhang, R. L. Liu, Y. Y. Liang and H. L. Li, J. Power Sources, 2008, 185, 801–806 CrossRef CAS PubMed.
- M. Carmo, M. Brandalise, A. O. Neto, E. V. Spinacé, A. D. Taylor, M. Linardi and J. G. Rocha Poço, Int. J. Hydrogen Energy, 2011, 36, 14659–14667 CrossRef CAS PubMed.
- G. Wu, Y. S. Chen and B. Q. Xu, Electrochem. Commun., 2005, 7, 1237–1243 CrossRef CAS PubMed.
- S. Yang, X. Zhang, H. Mi and X. Ye, J. Power Sources, 2008, 175, 26–32 CrossRef CAS PubMed.
- Z. P. Sun, X. G. Zhang, H. Tong, Y. Y. Liang and H. L. Li, J. Colloid Interface Sci., 2009, 337, 614–618 CrossRef CAS PubMed.
- Z. P. Sun, X. G. Zhang, Y. Y. Liang and H. L. Li, J. Power Sources, 2009, 191, 366–370 CrossRef CAS PubMed.
- D. He, S. Mu and M. Pan, Carbon, 2011, 49, 82–88 CrossRef CAS PubMed.
- H. Kim, W. Lee and D. Yoo, Electrochim. Acta, 2007, 52, 2620–2624 CrossRef CAS PubMed.
- N. Cheng, S. Mu, X. Chen, H. Lv, M. Pan and P. P. Edwards, Electrochim. Acta, 2011, 56, 2154–2159 CrossRef CAS PubMed.
- M. Carmo, T. Roepke, C. Roth, A. M. dos Santos, J. G. R. Poco and M. Linardi, J. Power Sources, 2009, 191, 330–337 CrossRef CAS PubMed.
- Z. Q. Zhiqiang Xu and A. Kaufmanb, Electrochem. Solid-State Lett., 2005, 8, A313–A315 CrossRef PubMed.
- S. Limpattayanate and M. Hunsom, J. Solid State Electrochem., 2013, 17, 1221–1231 CrossRef CAS.
- T. F. Hung, B. Wang, C. W. Tsai, M. H. Tu, G. X. Wang, R. S. Liu, D. P. Tsai, M. Y. Lo, D. S. Shy and X. K. Xing, Int. J. Hydrogen Energy, 2012, 37, 14205–14210 CrossRef CAS PubMed.
- D. He, Z. Kou, Y. Xiong, K. Cheng, X. Chen, M. Pan and S. Mu, Carbon, 2014, 66, 312–319 CrossRef CAS PubMed.
- H. Huang, W. Zhang, M. Li, Y. Gan, J. Chen and Y. Kuang, J. Colloid Interface Sci., 2005, 284, 593–599 CrossRef CAS PubMed.
- H. Gharibi, M. Faraji and M. Kheirmand, Electroanalysis, 2012, 24, 2354–2364 CrossRef CAS PubMed.
- H. Yu, Y. Jin, Z. Li, F. Peng and H. Wang, J. Solid State Chem., 2008, 181, 432–438 CrossRef CAS PubMed.
- E. Antolini, J. Appl. Electrochem., 2004, 34, 563–576 CrossRef CAS.
- F. Barroso-Bujans, J. L. G. Fierro, S. Rojas, S. Sánchez-Cortes, M. Arroyo and M. A. López-Manchado, Carbon, 2007, 45, 1669–1678 CrossRef CAS PubMed.
- T. F. Hung, S. H. Liao, C. Y. Li and Y. W. Chen-Yang, J. Power Sources, 2011, 196, 126–132 CrossRef CAS PubMed.
- Z. B. Wang, P. J. Zuo, X. P. Wang, J. Lou, B. Q. Yang and G. P. Yin, J. Power Sources, 2008, 184, 245–250 CrossRef CAS PubMed.
- A. Pozio, M. de Francesco, A. Cemmi, F. Cardellini and L. Giorgi, J. Power Sources, 2002, 105, 13–19 CrossRef CAS.
- H. Gharibi, F. Golmohammadi and M. Kheirmand, Electrochim. Acta, 2013, 89, 212–221 CrossRef CAS PubMed.
- J. Wu, X. Z. Yuan, H. Wang, M. Blanco, J. J. Martin and J. Zhang, Int. J. Hydrogen Energy, 2008, 33, 1735–1746 CrossRef CAS PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons, Inc., 2001 Search PubMed.
- H. Gharibi, M. Amani, H. Pahlavanzadeh and M. Kazemeini, Electrochim. Acta, 2013, 97, 216–225 CrossRef CAS PubMed.
- H. Gharibi, M. Javaheri and R. A. Mirzaie, Int. J. Hydrogen Energy, 2010, 35, 9241–9251 CrossRef CAS PubMed.
- H. Gharibi, K. Kakaei and M. Zhiani, J. Phys. Chem. C, 2010, 114, 5233–5240 CAS.
- J. M. K. Jae Hyung Cho, J. Prabhuram, S. Y. Hwang, H. Y. H. Dong June Ahn and S.-K. Kim, J. Power Sources, 2009, 187, 378–386 CrossRef PubMed.
- P. D. Beattie, V. I. Basura and S. Holdcroft, J. Electroanal. Chem., 1999, 468, 180–192 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09201a |
| This journal is © The Royal Society of Chemistry 2015 |





