Poly(sodium 4-styrenesulfonate) modified graphene for reinforced biodegradable poly(ε-caprolactone) nanocomposites†
Ming Wanga,
Xiao-Ying Denga,
An-Ke Du*b,
Tong-Hui Zhaoa and
Jian-Bing Zeng*a
aCollege of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, PR China. E-mail: jbzeng@swu.edu.cn; Fax: +86-23-68254000; Tel: +86-23-68254000
bChongqing Academy of Science and Technology, Chongqing 401123, China. E-mail: castduke@163.com
First published on 24th August 2015
Abstract
A homogeneous and stable water dispersion of graphene nanosheets (GNS) was prepared through a non-covalent functionalization technique by ultrasonic processing of GNS in the presence of poly(sodium 4-styrenesulfonate) (PSS) as the modifier. The dispersion was then used to compound with poly(ε-caprolactone) (PCL) through solution coagulation to fabricate PCL/GNS nanocomposites. Scanning and transmission electron microscope observations indicated that PSS modified GNS dispersed uniformly in and showed strong interfacial adhesion with the PCL matrix when the loading of GNS was not more than 0.5 wt%. While when the loading of GNS increased to 1.0 wt%, aggregation morphology of the GNS in the PCL matrix was detected. The crystallization temperature of PCL, as investigated using a differential scanning calorimeter, increased apparently by incorporation of PSS modified GNS. Investigation on isothermal crystallization kinetics of neat PCL and its composites indicated that the crystallization of PCL was accelerated considerably. Addition of only 0.05 wt% GNS caused a 5.8 times improvement in crystallization rate compared to neat PCL. The crystallization mechanism almost remained unchanged. The improvement in crystallization rate was ascribed to the enhanced nucleation density by incorporation of PSS modified GNS, as evidenced using a polarizing optical microscope (POM). Tensile tests showed that both the tensile strength and the Young's modulus of PCL were reinforced and increased gradually with increasing GNS loading within 0.5 wt%, meanwhile the elongation at break did not reduce but increased slightly. While when the loading of GNS increased to 1.0 wt%, the tensile strength and elongation at break reduced considerably due to the aggregation of GNS with increasing loading. Dynamic mechanical analysis indicated that the storage modulus of PCL was reinforced in the full temperature range by incorporation of PSS modified GNS.
1. Introduction
Graphene, as a one-atom-thick two-dimensional layer of sp2-bonded carbon, shows a lot of unique properties with many property parameters measured in experiments superior to any other material and even some reaching theoretical limits.1 For example, the room-temperature electron mobility is 2.5 × 105 cm2 V−1 s−1,2 approaching the theoretical limit of ∼2 × 105 cm2 V−1 s−1,3 the Young's modulus and intrinsic strength are 1 TPa and 130 GPa,4 respectively, very close to the predicted values.5 Besides, graphene shows very high thermal conductivity,6 complete impermeability to any gas,7 room-temperature quantum hall effect,8 room-temperature ferromagnetism,9 as well as many other supreme properties. Those properties make it highly attractive for numerous applications such as printable and flexible electronics, high-frequency transistors, photodetectors, optical modulators, supercapacitors, batteries, biomaterials, etc.1The incorporation of graphene into polymer matrix to form polymer nanocomposite represents an important application of this unique nanomaterial, as it has the potential to reinforce numerous properties of or impart some novel functionalities to matrix polymers.10,11 Therefore, extensive works have been done to incorporate graphene into various polymers and investigate the properties of the formed composites.12–16 The dispersion state of graphene plays a vital role in the final properties of the composites. Small addition could significantly reinforce many properties of host polymers if in which graphene dispersed uniformly. However, polymer composites with well dispersed graphenes are hard to achieve because graphenes have a pronounced tendency to agglomerate in polymer matrices due to the strong π–π interactions between graphene nanosheets.13 So, to improve and stabilize the dispersion state of graphene in host polymers constitutes the greatest challenge in graphene based polymer nanocomposites.
The efficient way to prevent graphenes from aggregation is to weaken the π–π interactions via either chemical modification or non-covalent functionalization.17 Chemical modification, also known as covalent functionalization, involves chemical reactions between modifiers and graphene or its derivatives that contain reactive functional groups, such as graphene oxide18–21 and reduced graphene oxide.22,23 The presence of surface grafted modifiers with different natures could disturb the π–π interactions among graphene sheets thus facilitate dispersion of graphene in various solvents or polymer matrices.13 It is however worth noting that the structural regularity of graphene is usually interrupted during chemical modification, which thus reduces the performances of graphene, for example, lowering electrical conductivity.24
By contrast, non-covalent functionalization provides a way of improving dispersion of graphene without disturbing its structure and the electronic network.17,25,26 This method refers to the modification through physical absorption of modifiers onto the surface of graphene so as to improve its dispersity in different solvents. The modifiers are usually the substances that are able to form some particular interactions, such as π–π, cation–π, anion–π interactions, with graphene sheets.17 Surfactants, such as sodium dodecylbenzene sulfonate (SDBS),27–29 sodium dodecyl sulfate (SDS),30 cetyltrimethylammonium bromide (CTAB),27,31 and 4-(1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol (Triton X-100)32 are the widely used non-covalent modifiers for graphene filled in polymer composites. In addition to surfactants, some polymers that can form special interactions with graphene were also used as non-covalent modifiers. Poly(sodium 4-styrenesulfonate) (PSS) was found to be able to prevent reduced graphene oxide from agglomeration upon reduction by hydrazine,33 and could be used to facilitate preparation of graphene through electrolytic exfoliation of graphite, as the aromatic rings of PSS could form edge-to-face interaction (π–π interaction) with graphene surface.34 It seems that PSS modified graphenes were often used to fabricate multilayer films through layer-by-layer technique35–37 but rarely employed to incorporate into polymer matrix to form conventional composites.38 As PSS modified graphenes are only dispersible in water, it is a challenge to incorporate such graphemes into water insoluble polymers.
Biodegradable aliphatic polyesters such as poly(lactic acid) (PLA) and poly(ε-caprolactone) (PCL) have attracted considerable attentions due to their eco-friendly nature.39–44 However, those polymers have more or less disadvantages that restrict their applications. Incorporation of graphene into biodegradable polymers provides an effective way of reinforcing their properties.45–49 Some papers focusing on PCL/graphene composites have been published.50–52 However, as the graphenes used in those studies were unmodified ones, which tended to agglomerate in PCL matrix. Therefore, further efforts are still required to improve the dispersion of graphene in PCL matrix to present high reinforcing efficiency. In this paper, we modify graphene with PSS by direct ultrasound of water suspension of graphene in the presence of PSS, then incorporate PSS modified graphene into PCL to form composites, and finally investigate the effect of loadings of PSS modified graphenes on the morphology, crystallization behaviors, and mechanical properties of the composites.
2. Experimental section
2.1. Materials
Poly(ε-caprolactone) (PCL, Esun500C) with molecular weight of 5.0 × 104 g mol−1 was purchased from Guanghua Weiye industrial co., Ltd (Shenzhen, China). Graphene nanosheets (GNSs) (NO: XF001W) was procured from XF NANO Materials Tech Co., Ltd (Nanjing, China). According to the manufacture claimed, the GNS was prepared by physical methods and has diameter of 0.5–2 μm, thickness of ∼0.8 nm, and single layer ratio of ∼80%. Poly(sodium 4-styrenesulfonate) (PSS, MW: 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) with 21 wt% aqueous solution was obtained from Micxy Chemical Co., Ltd (Chengdu, China). Tetrahydrofuran (THF) and other chemicals with AR grades were obtained from Kelong Chemical Co., Ltd (Chengdu, China). All of the raw materials were used without further purification.
000) with 21 wt% aqueous solution was obtained from Micxy Chemical Co., Ltd (Chengdu, China). Tetrahydrofuran (THF) and other chemicals with AR grades were obtained from Kelong Chemical Co., Ltd (Chengdu, China). All of the raw materials were used without further purification.
2.2. Modification of GNS with PSS
Modification of GNS with PSS was carried out by ultrasound of GNS water suspension containing PSS at room temperature for 30 min. The weight ratio of GNS to PSS was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and the concentration of GNS in water was 2.0 g L−1. The detailed description for the modification procedure is as follow: GNS water suspension was prepared by addition of 0.40 g GNSs, 9.52 g PSS solution, and 190.18 g H2O into a 500 mL beaker; the suspension was then sonicated with a probe sonicator (SCIENTZ-IID, Ningbo China) for 30 min to form a uniform dispersion. The dispersion was used directly for preparation of PCL/GNS composites. For comparison, GNSs water dispersion without PSS was also prepared by the similar processing.
5, and the concentration of GNS in water was 2.0 g L−1. The detailed description for the modification procedure is as follow: GNS water suspension was prepared by addition of 0.40 g GNSs, 9.52 g PSS solution, and 190.18 g H2O into a 500 mL beaker; the suspension was then sonicated with a probe sonicator (SCIENTZ-IID, Ningbo China) for 30 min to form a uniform dispersion. The dispersion was used directly for preparation of PCL/GNS composites. For comparison, GNSs water dispersion without PSS was also prepared by the similar processing.
2.3. Preparation of PCL/GNS composites
PCL/GNS composites with GNS loading varying from 0.05 to 1.0 wt% were prepared through a solution coagulation technique. THF was used to dissolve PCL and the concentration was ∼5% (g/vol). Taking preparation of the composite with GNS loading of 0.1 wt% for an example, the procedures were: 9.99 g PCL was dissolved in 200 mL THF with mild stirring at 50 °C for 2 h; after cooling down to room temperature, 5 g of above prepared GNS dispersion was dropped into the strongly stirred PCL/THF solution, then excessive H2O was dropped to coagulate the composites, which were collected by filtration, washed with ethanol, and dried in a 50 °C air blast oven to evaporate solvents, and finally vacuum dried at 50 °C for 48 h to remove any residual solvent. For convenience, we abbreviated the composite to PCL/GNS-x, where x represents the loading of the GNS. For example, PCL/GNS-0.1 indicates the composite containing 0.1 wt% GNS. For comparison, neat PCL was also processed with the similar procedures. The sample sheets with thickness of 1 mm were prepared by hot pressing for further characterization.2.4. Characterization
The morphologies of original GNS and PSS modified GNS were observed on a JEM-2100 transmission electron microscope (TEM) an accelerating voltage of 200 kV. Samples were prepared by depositing a drop of the water dispersion onto a copper micro grid and then vacuum dried at 80 °C for 24 h.The morphologies for the cryo-fractured surfaces of PCL/GNS nanocomposites were observed by a XL-30 s FEG (Philips, Holland) scanning electron microscope (SEM) with an accelerating voltage of 5 kV. The fractured surface was sputtered with a layer of gold prior to observation.
The dispersion of PSS modified GNS in PCL matrix was observed by a JEM-2100F transmission electron microscope with an accelerating voltage of 200 kV. Ultrathin sections of ca. 70–80 nm in thickness were sliced using a Leica EM FC6 cryo-ultramicrotome.
Thermal and crystallization behaviors of neat PCL and its composites were investigated by a NETZSCH DSC-214 differential scanning calorimeter. The samples with ∼6 mg in aluminum pans were first melted at 80 °C for 3 min to remove thermal history, then cooled to −60 °C at a scanning rate of 10 °C min−1, and finally reheated to 80 °C at the same scanning rate. All the operations were carried out under N2 atmosphere. Both the cooling and the second heating scans were recorded for data analysis.
Isothermal crystallization kinetics was conducted on the NETZSCH DSC-214 differential scanning calorimeter. The samples with ∼6 mg in aluminum pans were first melted at 80 °C for 3 min to remove thermal history, and then quickly cooled to 44 °C at a cooling rate of 60 °C min−1, and finally kept at 44 °C until crystallization completed. The operations were carried out under N2 atmosphere. The crystallization exothermal curves were recorded for analysis.
Spherulitic morphologies of neat PCL and its composites were investigated by a NIKON ECLIPSE LV100POL polarizing optical microscope with an HSC621V temperature controller. The sample films in two microscopic cover glasses were first melted at 80 °C for 3 min to remove any thermal history and then rapidly cooled down to 40 °C and kept at this temperature until crystallization finished.
X-ray diffraction patterns of neat PCL and its composites were recorded with an X-ray diffractometer (Philips X'Pert X-ray diffractometer) with Cu Kα radiation. The equipment was operated at room temperature with a scanning rate of 2° min−1 scanning from 5 to 40°.
The mechanical properties neat PCL and its composites were measured on a Sansi CMT6503 Universal Testing Machine at a crosshead speed of 50 mm min−1 at room temperature. The dumbbell-shaped specimen with respective width and thickness of 4 and 1 mm were used. The length between the two mechanical grips of the testing machine was 25 mm. At least five specimens were tested for each sample, and the averaged result was reported.
Thermo-mechanical properties of neat PCL and its composites were tested on a TA DMA Q800 dynamic mechanical analyzer using a tensile mode. Tests were performed from −70 to 40 °C at a heating rate of 3 °C min−1 and an oscillation frequency of 1 Hz.
3. Results and discussion
3.1. Preparation and morphologies of PSS modified GNS reinforced PCL composites
GNSs were modified with PSS under the aid of ultrasound prior to fabrication of PCL/GNS composites. The modification was done by probe ultrasonication of GNS/PSS (w/w, 1/5) water dispersion for 30 min. To make a comparison, water dispersion of original GNS (OGNS) was also treated with the same procedure. Fig. 1a shows the digital photos of water dispersions of OGNS and PSS modified GNS (MGNS). It seems that homogeneous water dispersions were obtained for both GNSs. However, the GNS particles adhered to the wall of bottle revealed the poor dispersion of OGNS in water. In contrast, the wall for the bottle that contained PSS modified GNS was very cleaning, indicating good dispersion of modified GNS in water. After placement for 24 h, original GNS went down to the bottom of the bottle as shown in Fig. 1b, revealing the instability of OGNS water dispersion, which was ascribed to the aggregation of graphene sheets arisen from their strong π–π interaction. In the case of PSS modified GNS, no obvious precipitate could be observed, indicative of good stability of the dispersion. The ability of PSS to stabilize GNS water dispersion was attributed to the formation of π–π interaction between benzene rings of PSS and graphene sheet, which consequently reduced the interactions among graphene sheets and thus prevented them from aggregation. The morphologies of OGNS and MGNS were characterized by TEM. Fig. 1c and d shows the TEM images of OGNS and MGNS, respectively. Original GNS showed typical characteristic of graphene sheets, which aggregated strongly. In addition, many folds could be found. For PSS modified GNS, although it was hard to detect the surface wrapped PSS, the aggregation of graphene sheets was reduced significantly and the density of folds was much lower than that of OGNS, suggesting that the presence of PSS was very efficient to stop graphene sheets from agglomeration. The homogeneous and stable MGNS dispersion should be used as a useful material to incorporate GNS into polymer matrix via a solution compounding technique to achieve a well-dispersed morphology and improved physical properties. | ||
| Fig. 1 Digital photos for the as prepared water dispersions of original GNS (OGNS) and PSS modified GNS (MGNS) (a) and for the dispersions placed for 24 h (b); TEM images for OGNS (c) and MGNS (d). | ||
As PSS modified GNS is only dispersible in aqueous medium due to the hydrophilic nature of PSS, it seem hard to incorporate it into water insoluble or dispersible polymers due to their different solubility. However, solution coagulation provides an alternative way of compounding PSS modified GNS with water insoluble polymers if they can dissolve in a solvent that is miscible with water. Therefore, we incorporated PSS modified GNS into PCL matrix which was dissolved in THF. A series of PSS modified GNS filled PCL composites containing various loadings of GNS were prepared through solution coagulation. For brevity, the composite was abbreviated as PCL/GNS-x, where x represents the loading of GNS in percentage. For example, PCL/GNS-0.05 represents PSS modified GNS filled PCL composite that contains 0.05 wt% GNS. Due to the poor dispersity of original GNS in water, we did not prepare original GNS filled PCL composite.
It is well-known that the homogeneous dispersion of GNS in polymer matrix plays an important role in determining final properties of the composite. The morphologies of neat PCL and its composites were observed by SEM. Fig. 2 shows the SEM images of cryo-fractured surfaces of neat PCL, PCL/GNS-0.05, PCL/GNS-0.5, and PCL/GNS-1.0. The cryo-fractured surface of neat PCL as shown in Fig. 2a was smooth, while those of PCL/GNS composites became more buckling with increasing loading of PSS modified GNS. However, it is worth noting that neither apparent agglomeration nor pulling-out of GNS from PCL matrix was observed when the loading of GNS was not more than 0.5 wt% (Fig. 2b and c), indicating a strong interfacial interaction between PSS modified GNS and PCL matrix, which could be attributed to the hydrophilic character of PCL and PSS modified GNS. It is worth noting that although PCL is water insoluble, it is hydrophilic polyester as it can absorb up to 1.0 wt% water molecules.53 When the content of GNS increased to 1.0 wt%, the fractured surface of the composite showed some aggregations due to agglomeration of GNS as shown in Fig. 2d.
 | ||
| Fig. 2 SEM images for cryo-fractured surfaces of neat PCL (a), PCL/GNS-0.05 (b), PCL/GNS-0.5 (c), and PCL/GNS-1.0 (d). | ||
TEM was employed to further observe the effect of loadings on the dispersion state of PSS modified GNS in PCL matrix. Fig. 3 shows the TEM images of PCL/GNS-0.5 and PCL/GNS-1.0. The low magnified image of microtomic specimen showed that GNS dispersed uniformly in PCL matrix for PCL/GNS-0.5 (Fig. 3a). In the case of PCL/GNS-1.0, some obvious aggregations of GNS could be observed, as shown in Fig. 3c. The high magnified TEM images (Fig. 3b and d) revealed that GNS showed a characteristic worm-like morphology with some folds in PCL matrix. Agglomeration of GNS was not found for PCL/GNS-0.5 (Fig. 3b) but detected for PCL/GNS-1.0 (Fig. 3d), which is in agreement with the results observed by SEM.
 | ||
| Fig. 3 TEM images for PCL/GNS-0.5 (a and b) and PCL/GNS-1.0 (c and d) with low magnification (a and c) and high magnification (b and d). | ||
XRD is a good tool to detect the stacking structure of graphenes in the nanocomposites.54 The diffraction peak for layer-to-layer distance of layered graphenes usually existed at around 2θ of 10°.55,56 Fig. 4 shows the XRD diffractions of neat PCL and its nanocomposites. It is clear that no diffraction peak can be observed below 2θ of 15° for all samples, which indicate that the stacking structure of the GNS powders was disordered in the PCL composites.55 Neat PCL showed three typical diffraction peaks at 2θ = 21.27, 21.87, and 23.55°, corresponding to (110), (111), and (200) planes,52 respectively. It is worth noting that the composites also showed the same three diffraction peaks, indicating that the incorporation of PSS modified GNS does not change the crystal structure of PCL.
3.2. Thermal and crystallization behaviors
DSC was used to study the effect of GNS loadings on the thermal and crystallization behaviors of PCL/GNS composites. Fig. 5 shows the DSC cooling scans and the second heating scans of the samples at scanning rates of 10 °C min−1. All the samples crystallized during cooling scan. Neat PCL shows a wide crystallization exothermic peak with a crystallization temperature (Tc) of 25.5 °C. The crystallization exothermic peak was narrowed by incorporation of PSS modified GNS and shifted to higher temperature range, indicating improved crystallization rate possible due to enhanced nucleation efficiency with addition of GNS. For the effect of GNS loadings on the crystallization of the composites, it is interesting to find that the Tc of PCL increased significantly to 33 °C with addition of only 0.05 wt% GNS, suggesting good nucleation efficiency. Similar improvement in crystallization temperature was obtained with addition of about ten times (0.5 wt%) amount of unmodified reduced graphene oxide, as reported by Wang et al.50 and Zhang and Qiu.52 With further increasing GNS loadings, the Tc increased slightly. The values were 33.3, 34.4, 35.3, and 35.8 °C for PCL/GNS-0.1, PCL/GNS-0.3, PCL/GNS-0.5, and PCL/GNS-1.0, respectively. The enthalpies of crystallization for all samples were around −60 J g−1. It is noted that glass transition of PCL could not be observed from the second heating scans, which was ascribed to the fast crystallization rate of the samples and the limitation for the mechanical refrigeration system of DSC 214. All the samples showed almost the same melting temperatures of around 58 °C and the same fusion enthalpies of around 60 J g−1, which indicated that the incorporation of GNS did not change the crystal structure and the degree of crystallinity of PCL. From those results, we can conclude that the incorporation of PSS modified GNS did not change the melting temperature and degree of crystallinity of PCL but accelerated the crystallization rate of PCL by nucleation. | ||
| Fig. 5 DSC cooling (a) and the second heating scans (b) of neat PCL and its composites at scanning rate of 10 °C min−1. | ||
In order to study the effect of GNS loadings on the crystallization behaviors of PCL composites in detail, the isothermal crystallization of neat PCL, PCL/GNS-0.05, PCL/GNS-0.5, and PCL/GNS-1.0 at 44 °C were carried out and analyzed by Avrami equation. Fig. 6a shows the development of relative crystallinity (Xt) with crystallization time at crystallization temperature of 44 °C for neat PCL and its composites. Neat PCL finished crystallization in ∼45 min. Incorporation of 0.05 wt% GNS, the crystallization developed quickly and finished in ∼7 min. When the loading of GNS increased to 0.5 wt%, the time required to complete crystallization further reduced to 3.3 min. While with further increased loadings of GNS to 1.0 wt%, the time only reduced to 3.2 min. The results indicate that the crystallization rate of PCL increased significantly with increasing GNS loading within 0.5 wt% while almost remained with further increasing GNS loading.
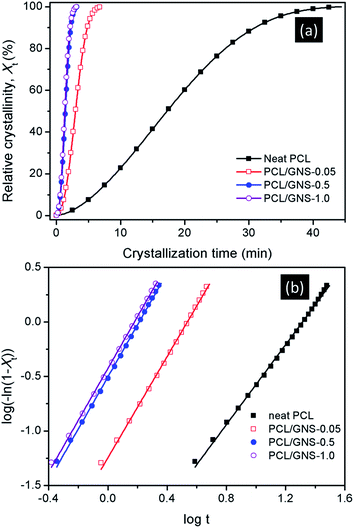 | ||
| Fig. 6 (a) Development of relative crystallinity with time and (b) Avrami plots for isothermal crystallization of PCL and its composites at 44 °C. | ||
The isothermal crystallization kinetics of neat PCL and its composites were analyzed by the Avrami equation:57–59
| 1 − Xt = exp(−ktn) |
log[−ln(1 − Xt)] = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k + n k + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t t |
A plot of log[−ln(1 − Xt)] versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t would give a straight line from which both the rate constant and the Avrami exponent can be derived. Fig. 6b shows the Avrami plots of neat PCL and its composites at crystallization temperature of 44 °C. The respective n value for neat PCL, PCL/GNS-0.05, PCL/GNS-0.5, and PCL/GNS-1.0 was 1.83, 2.32, 2.39, and 2.38. The results reveal that polymeric chains in both neat PCL and its composites adopt a two-dimensional crystallization growth with a heterogeneous type of nucleation, in agreement with other studies focused on crystallization kinetics of PCL.60–62 The crystallization rate constants were 3.90 × 10−3, 5.68 × 10−2, 0.31, and 0.37 min−n. It seems unreasonable to compare the crystallization rates of the samples from their rate constants since the n values were also changing. The reciprocal of half-time of crystallization (1/t1/2) can be used directly to describe the overall crystallization rate of the sample. The 1/t1/2 values of neat PCL for isothermal crystallization at 44 °C was only 0.058 min−1. The overall crystallization rate was increased by 5.8 times to 0.336 min−1 upon addition of only 0.05 wt% GNS. When 0.5 and 1.0 wt% GNSs were incorporated, the overall crystallization rates were increased by 12.2 and 13.3 times, with the values of 0.709 and 0.769 min−1, respectively.
t would give a straight line from which both the rate constant and the Avrami exponent can be derived. Fig. 6b shows the Avrami plots of neat PCL and its composites at crystallization temperature of 44 °C. The respective n value for neat PCL, PCL/GNS-0.05, PCL/GNS-0.5, and PCL/GNS-1.0 was 1.83, 2.32, 2.39, and 2.38. The results reveal that polymeric chains in both neat PCL and its composites adopt a two-dimensional crystallization growth with a heterogeneous type of nucleation, in agreement with other studies focused on crystallization kinetics of PCL.60–62 The crystallization rate constants were 3.90 × 10−3, 5.68 × 10−2, 0.31, and 0.37 min−n. It seems unreasonable to compare the crystallization rates of the samples from their rate constants since the n values were also changing. The reciprocal of half-time of crystallization (1/t1/2) can be used directly to describe the overall crystallization rate of the sample. The 1/t1/2 values of neat PCL for isothermal crystallization at 44 °C was only 0.058 min−1. The overall crystallization rate was increased by 5.8 times to 0.336 min−1 upon addition of only 0.05 wt% GNS. When 0.5 and 1.0 wt% GNSs were incorporated, the overall crystallization rates were increased by 12.2 and 13.3 times, with the values of 0.709 and 0.769 min−1, respectively.
It is well-known that the crystallization rate of a polymer is dependent on the nucleation density and crystal growth rate. In this study, the incorporation of GNS is unable to increase the crystal growth rate of PCL as the layered structure of GNS may confine the mobility of polymer chains.63 Thus the improved crystallization rate could only be ascribed to the improved nucleation density by incorporation of GNS. POM was used to observe the crystalline morphologies of neat PCL and its composites. Fig. 7 shows the spherulitic morphology of neat PCL and its composites formed by isothermal crystallization at 40 °C. Large spherulites with low nucleation density were observed for neat PCL. The size of spherulites decreased while the number of spherulites increased significantly with addition of 0.05 wt% GNS, indicating significantly improved nucleation density. With further increasing loadings of GNS to 0.5 and 1.0 wt%, a large number of small-sized crystals were formed the regular spherulites were hard to distinguished, revealing further increased nucleation density. From above discussion, we can conclude that incorporation of PSS modified GNS was capable of accelerating crystallization rate of PCL by improving nucleation density without changing the crystallization mechanism.
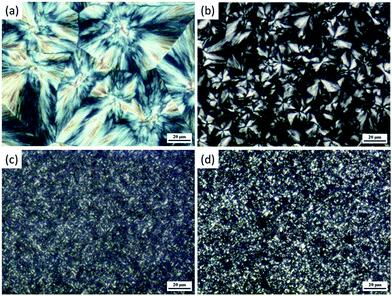 | ||
| Fig. 7 Spherulitic morphologies of neat PCL (a), PCL/GNS-0.05, PCL/GNS-0.5, and PCL/GNS-1.0 formed by isothermal crystallization at 40 °C. | ||
3.3. Mechanical properties
GNSs with unique mechanical strength and modulus can be used as ideal fillers for reinforcing mechanical properties of polymer matrix. The static mechanical properties of neat PCL and PCL/GNS composites were measured by tensile test to study the effect of GNS loadings on the yield strength, Young's modulus, and elongation at break of PCL composites. The results are summarized in Table 1. The yield strength and Young's modulus of neat PCL were 17.2 and 298.2 MPa, respectively. Both the yield strength and the Young's modulus showed uptrends with increasing GNS loading within 0.5 wt%, revealing reinforcement. Both yield strength and Young's modulus of PCL were improved by ∼12% when the loading of GNS was only 0.5 wt%. PSS modified graphenes showed better reinforcing efficiency in yield strength than unmodified reduced graphene oxides (RGOs) with same loading, incorporation of 0.5 wt% RGOs cause 1.1 MPa in yield strength of PCL, as reported by Wang et al.50 Furthermore, it is worth noting that the elongation at break was not reduced but also increased when the loading of GNS was not more than 0.5 wt%. The improvement in the mechanical properties should be ascribed to the good dispersion of PSS modified GNS in PCL matrix as well as the strong interfacial adhesion between PSS modified GNS and PCL matrix which is helpful for stress transfer from low strength PCL matrix to high strength GNS fillers to reinforce the mechanical properties of the composites. When the loading of GNS increased to 1.0 wt%, although the Young's modulus were further increased, the yield strength was reduced slightly and elongation at break was dropped considerable, which might be ascribed to the aggregation of GNS as discussed in Section 3.1.| Sample | Yield strength (MPa) | Young's modulus (MPa) | Elongation at break (%) |
|---|---|---|---|
| Neat PCL | 17.2 ± 0.2 | 298.2 ± 10.4 | 530 ± 17 |
| PCL/GNS-0.05 | 18.6 ± 0.7 | 305.0 ± 4.2 | 597 ± 30 |
| PCL/GNS-0.1 | 18.9 ± 1.1 | 309.4 ± 18.8 | 584 ± 31 |
| PCL/GNS-0.3 | 19.0 ± 1.0 | 324.6 ± 22.6 | 558 ± 22 |
| PCL/GNS-0.5 | 19.4 ± 0.8 | 333.5 ± 3.2 | 600 ± 10 |
| PCL/GNS-1.0 | 17.9 ± 1.3 | 360.5 ± 4.2 | 10 ± 2 |
Besides static mechanical properties, dynamic mechanical properties were also investigated to evaluate the effect of GNS loading on the storage modulus of the composites. The dynamic storage moduli of neat PCL and its composites were measured by dynamic mechanical analyzer. Fig. 8a shows the dynamic storage modulus (E′) as a function of temperature for neat PCL and the composites. It can be seen from the storage modulus plots that all samples showed α-relaxations at around −40 °C (i.e., glass transition temperature), and corresponding relaxation peak can be observed on the tan delta plots (Fig. 8b). Drastic drop in storage modulus occurred at around glass transition temperature of PCL. It is worth noting that all PCL/GNS composites showed higher storage moduli than neat PCL in the full temperature range, indicating reinforcement by incorporation of PSS modified GNS. The loading of GNS plays an important role in the storage modulus of the composite, which showed uptrend with increasing GNS loading. For example, the E′ value below glass transition (∼−40 °C) for neat PCL at −60 °C was 2633 MPa. With only 0.05 wt% GNS incorporated, the value increased to 2883 MPa; and with the GNS loadings increased to 0.5 and 1.0 wt%, the storage modulus further increased to 3129 and 3214 MPa, improving by 18.8% and 22.1%, respectively, compared to neat PCL. The E′ values above glass transition such as at 0 °C were 600 MPa for neat PCL, which increased to 651, 721, and 830 MPa for PCL/GNS-0.05, PCL/GNS-0.5, and PCL/GNS-1.0, showing improvement in percentage of 8.5, 20.2, and 38.3%, respectively.
4. Conclusions
Ultrasonication of GNS in the presence of PSS water solution gives rise to a homogeneous and stable dispersion, which could be then used to compound with PCL through a solution coagulation technique to prepare PCL/GNS nanocomposites. It was found that GNS dispersed almost uniformly in PCL matrix without apparent aggregation when the loading of GNS was not more than 0.5 wt% and showed strong interfacial adhesion with PCL. However, when the loading increased to 1.0 wt%, aggregation of GNS occurred. The crystallization of PCL was accelerated significantly by incorporation of PSS modified GNS. Addition of only 0.05 wt% GNS caused 5.8 times improvement in overall crystallization rate, which increased by 12.2 times with GNS loading increased to 0.5 wt%. While with further increasing GNS loading the improving extent in crystallization rate became very small. The acceleration in crystallization was ascribed to the significantly improved nucleation density by incorporation of GNS. The tensile strength and Young's modulus increased gradually with increasing PSS modified GNS loading within 0.5 wt%, and the elongation at break did not reduce but increased, due to the good dispersion and strong interfacial adhesion of the composites. But when the loading of GNS increased to 1.0 wt%, the tensile strength and elongation at break deteriorated considerably ascribing to the aggregation of GNS. The incorporation of PSS modified GNS resulted in reinforced dynamic storage modulus in the full temperature range. PCL/GNS-0.5 showed ∼18% and ∼20% improvement in storage modulus below Tg (−60 °C) and above Tg (0 °C), respectively, compared to neat PCL.Acknowledgements
This work was supported by Fundamental Research Funds for the Central Universities (XDJK2015C022) and the Natural Science Foundation Project of Chongqing (CSTC2013JCYJA20017).References
- K. S. Novoselov, V. I. Fal'ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192–200 CrossRef CAS PubMed.
- A. S. Mayorov, R. V. Gorbachev, S. V. Morozov, L. Britnell, R. Jalil, L. A. Ponomarenko, P. Blake, K. S. Novoselov, K. Watanabe, T. Taniguchi and A. K. Geim, Nano Lett., 2011, 11, 2396–2399 CrossRef CAS PubMed.
- S. V. Morozov, K. S. Novoselov, M. I. Katsnelson, F. Schedin, D. C. Elias, J. A. Jaszczak and A. K. Geim, Phys. Rev. Lett., 2008, 100, 016602 CrossRef CAS.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- F. Liu, P. Ming and J. Li, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 064120 CrossRef.
- A. A. Balandin, Nat. Mater., 2011, 10, 569–581 CrossRef CAS PubMed.
- J. S. Bunch, S. S. Verbridge, J. S. Alden, A. M. van der Zande, J. M. Parpia, H. G. Craighead and P. L. McEuen, Nano Lett., 2008, 8, 2458–2462 CrossRef CAS PubMed.
- K. S. Novoselov, Z. Jiang, Y. Zhang, S. V. Morozov, H. L. Stormer, U. Zeitler, J. C. Maan, G. S. Boebinger, P. Kim and A. K. Geim, Science, 2007, 315, 1379 CrossRef CAS PubMed.
- Y. Wang, Y. Huang, Y. Song, X. Zhang, Y. Ma, J. Liang and Y. Chen, Nano Lett., 2009, 9, 220–224 CrossRef CAS PubMed.
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666–686 RSC.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
- K. Hu, D. D. Kulkarni, I. Choi and V. V. Tsukruk, Prog. Polym. Sci., 2014, 39, 1934–1972 CrossRef CAS PubMed.
- T. Kuilla, S. Bhadra, D. Yao, N. H. Kim, S. Bose and J. H. Lee, Prog. Polym. Sci., 2010, 35, 1350–1375 CrossRef CAS PubMed.
- Z. Jiang and Z. Qiu, RSC Adv., 2015, 5, 55486–55491 RSC.
- R. B. Valapa, G. Pugazhenthi and V. Katiyar, RSC Adv., 2015, 5, 28410–28423 RSC.
- Y. Zhou, H. Xiu, J. Dai, H. Bai, Q. Zhang and Q. Fu, RSC Adv., 2015, 5, 30912–30919 RSC.
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156–6214 CrossRef CAS PubMed.
- S. Stankovich, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Carbon, 2006, 44, 3342–3347 CrossRef CAS PubMed.
- Y. Sun and C. He, ACS Macro Lett., 2012, 1, 709–713 CrossRef CAS.
- D. D. Zhang, S. Z. Zu and B. H. Han, Carbon, 2009, 47, 2993–3000 CrossRef CAS PubMed.
- F. Y. Yuan, H. B. Zhang, X. F. Li, H. L. Ma, X. Z. Li and Z. Z. Yu, Carbon, 2014, 68, 653–661 CrossRef CAS PubMed.
- T. Phung Xuan, C. Basavajara, J. K. Kim and D. S. Huh, Polym. Compos., 2012, 33, 2159–2168 CrossRef PubMed.
- P. Ding, S. S. Su, N. Song, S. F. Tang, Y. M. Liu and L. Y. Shi, Carbon, 2014, 66, 576–584 CrossRef CAS PubMed.
- E. E. Tkalya, M. Ghislandi, G. de With and C. E. Koning, Curr. Opin. Colloid Interface Sci., 2012, 17, 225–231 CrossRef CAS PubMed.
- J. R. Lomeda, C. D. Doyle, D. V. Kosynkin, W.-F. Hwang and J. M. Tour, J. Am. Chem. Soc., 2008, 130, 16201–16206 CrossRef CAS PubMed.
- M. Lotya, Y. Hernandez, P. J. King, R. J. Smith, V. Nicolosi, L. S. Karlsson, F. M. Blighe, S. De, Z. Wang, I. T. McGovern, G. S. Duesberg and J. N. Coleman, J. Am. Chem. Soc., 2009, 131, 3611–3620 CrossRef CAS PubMed.
- K. Zhang, L. Mao, L. L. Zhang, H. S. O. Chan, X. S. Zhao and J. S. Wu, J. Mater. Chem., 2011, 21, 7302–7307 RSC.
- L. Liu, B. Gao, L. Wu, Y. Sun and Z. Zhou, Chem. Eng. J., 2015, 262, 1187–1191 CrossRef CAS PubMed.
- X. Zhao, Q. H. Zhang, D. J. Chen and P. Lu, Macromolecules, 2010, 43, 2357–2363 CrossRef CAS.
- M. Moradi, J. A. Mohandesi and D. F. Haghshenas, Polymer, 2015, 60, 207–214 CrossRef CAS PubMed.
- C. F. Matos, F. Galembeck and A. J. G. Zarbin, Carbon, 2014, 78, 469–479 CrossRef CAS PubMed.
- M. E. Uddin, T. Kuila, G. C. Nayak, N. H. Kim, B. C. Ku and J. H. Lee, J. Alloys Compd., 2013, 562, 134–142 CrossRef CAS PubMed.
- S. Stankovich, R. D. Piner, X. Q. Chen, N. Q. Wu, S. T. Nguyen and R. S. Ruoff, J. Mater. Chem., 2006, 16, 155–158 RSC.
- G. Wang, B. Wang, J. Park, Y. Wang, B. Sun and J. Yao, Carbon, 2009, 47, 3242–3246 CrossRef CAS PubMed.
- S. Liu, J. Ou, Z. Li, S. Yang and J. Wang, Appl. Surf. Sci., 2012, 258, 2231–2236 CrossRef CAS PubMed.
- J. Luo, Y. Chen, Q. Ma, R. Liu and X. Liu, RSC Adv., 2013, 3, 17866–17873 RSC.
- Z. Li, J. Wang, X. Liu, S. Liu, J. Ou and S. Yang, J. Mater. Chem., 2011, 21, 3397–3403 RSC.
- Y. Li, J. Tang, L. Huang, Y. Wang, J. Liu, X. Ge, S. C. Tjong, R. K. Y. Li and L. A. Belfiore, Composites, Part A, 2015, 68, 1–9 CrossRef CAS PubMed.
- G. C. Liu, Y. S. He, J. B. Zeng, Q. T. Li and Y. Z. Wang, Biomacromolecules, 2014, 15, 4260–4271 CrossRef CAS PubMed.
- G. C. Liu, Y. S. He, J. B. Zeng, Y. Xu and Y. Z. Wang, Polym. Chem., 2014, 5, 2530–2539 RSC.
- M. Okada, Prog. Polym. Sci., 2002, 27, 87–133 CrossRef CAS.
- Y. S. He, J. B. Zeng, G. C. Liu, Q. T. Li and Y. Z. Wang, RSC Adv., 2014, 4, 12857–12866 RSC.
- S. Kumar, S. Bose and K. Chatterjee, RSC Adv., 2014, 4, 19086–19098 RSC.
- C. C. Neikirk, J. W. Chung and R. D. Priestley, RSC Adv., 2013, 3, 16686–16696 RSC.
- Z. Qiu and W. Guan, RSC Adv., 2014, 4, 9463–9470 RSC.
- X. Jing and Z. Qiu, Ind. Eng. Chem. Res., 2014, 53, 498–504 CrossRef CAS.
- X. Jing and Z. Qiu, Ind. Eng. Chem. Res., 2012, 51, 13686–13691 CrossRef CAS.
- H. Wang and Z. Qiu, Thermochim. Acta, 2012, 527, 40–46 CrossRef CAS PubMed.
- H. Wang and Z. Qiu, Thermochim. Acta, 2011, 526, 229–236 CrossRef CAS PubMed.
- B. Wang, Y. Li, G. Weng, Z. Jiang, P. Chen, Z. Wang and Q. Gu, Compos. Sci. Technol., 2014, 96, 63–70 CrossRef CAS PubMed.
- B. Wang, Y. Zhang, J. Zhang, H. Li, P. Chen, Z. Wang and Q. Gu, Ind. Eng. Chem. Res., 2013, 52, 15824–15828 CrossRef CAS.
- J. Zhang and Z. Qiu, Ind. Eng. Chem. Res., 2011, 50, 13885–13891 CrossRef CAS.
- P. Musto, M. Galizia, M. Pannico, G. Scherillo and G. Mensitieri, J. Phys. Chem. B, 2014, 118, 7414–7429 CrossRef CAS PubMed.
- Z. Wang, J. K. Nelson, H. Hillborg, S. Zhao and L. S. Schadler, Adv. Mater., 2012, 24, 3134–3137 CrossRef CAS PubMed.
- X. Wang, H. Yang, L. Song, Y. Hu, W. Xing and H. Lu, Compos. Sci. Technol., 2011, 72, 1–6 CrossRef CAS PubMed.
- X. Lu, J. Huang, L. Yang, N. Zhang, G. Jin and J. Qu, Polym. Adv. Technol., 2014, 25, 1515–1522 CrossRef CAS PubMed.
- M. Avrami, J. Chem. Phys., 1939, 7, 1103–1112 CrossRef CAS PubMed.
- M. Avrami, J. Chem. Phys., 1940, 8, 212–224 CrossRef CAS PubMed.
- M. Avrami, J. Chem. Phys., 1941, 9, 177–184 CrossRef CAS PubMed.
- G. Siqueira, C. Fraschini, J. Bras, A. Dufresne, R. Prud'homme and M. P. Laborie, Eur. Polym. J., 2011, 47, 2216–2227 CrossRef CAS PubMed.
- L. N. Luduena, A. Vazquez and V. A. Alvarez, J. Appl. Polym. Sci., 2008, 109, 3148–3156 CrossRef CAS PubMed.
- M. J. Jenkins and K. L. Harrison, Polym. Adv. Technol., 2006, 17, 474–478 CrossRef CAS PubMed.
- H. D. Huang, J. Z. Xu, Y. Fan, L. Xu and Z. M. Li, J. Phys. Chem. B, 2013, 117, 10641–10651 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15252f |
| This journal is © The Royal Society of Chemistry 2015 |


