Behavioral study of flexible platinum counter electrodes under alternative bending conditions†
Xue-Long Heab,
Ruo-Xi Shena,
Guan-Jun Yang*a,
Chang-Jiu Lia and
Baizeng Fang*c
aState Key Laboratory for Mechanical Behavior of Materials, School of Materials Science and Engineering, Xi'an Jiaotong University, Xi'an, Shaanxi 710049, PR China. E-mail: ygj@mail.xjtu.edu.cn; Tel: +86-29-82665299
bJiangxi Province Key Laboratory of Precision Drive and Control, Nanchang 330099, PR China
cDepartment of Chemical & Biological Engineering, University of British Columbia, 2360 East Mall, Vancouver, B.C., Canada V6T 1Z3. E-mail: bfang@chbe.ubc.ca
First published on 20th August 2015
Abstract
The electrocatalytic behavior of flexible platinum (Pt) counter electrodes (CEs) is of great importance to practical applications of flexible dye-sensitized solar cells (DSCs). In this study, the electrocatalytic activity of two different flexible Pt CEs with continuous and non-continuous Pt films, which were prepared by a sputtering method and an electroless deposition method, respectively, was studied under alternative bending conditions. The data obtained from electrochemical impedance spectroscopy measurements show that the electrocatalytic activity of the continuous Pt CEs slightly increases with the increasing bending cycles, due to the enhanced bare surface area formed from localized delamination and cracking. In contrast, the non-continuous Pt CEs demonstrate much higher resistance towards bending tests, i.e., their electrocatalytic activity remains unchanged even after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bending cycles. This can be attributed to the high strain tolerance and unique surface structure for this kind of non-continuous Pt CE, which helps the release of stress during the bending tests.
000 bending cycles. This can be attributed to the high strain tolerance and unique surface structure for this kind of non-continuous Pt CE, which helps the release of stress during the bending tests.
1. Introduction
The flexibility, as well as efficiency, of flexible dye-sensitized solar cells (DSCs) is of significant importance to their industrial applications.1–3 The counter electrode (CE) is one of the indispensable components in DSCs, which receives electrons from the external circuit and regenerates I− from I3− ions by catalysis.4–9 In this regard, both the electrocatalytic activity and bending resistance are equally important for flexible CEs.Due to its superior chemical and electrochemical stabilities, and electrocatalytic activity Pt has been widely used as an electrode material in electrochemical energy storage and conversion applications such as fuel cells, electrochemical hydrogen evolution, DSCs and quantum-dot SCs.10–15 To date, Pt is still the best candidate for flexible CEs.16 Many efforts have been paid to fabricate flexible Pt CEs with high electrocatalytic activity, such as sputtering,17–20 electroplating,21,22 chemical reduction23,24 and electroless deposition.25,26 However, to our best knowledge, there are few reports concerning the bending resistance of the flexible Pt CEs.
In this study, the electrocatalytic activity and bending resistance of the flexible Pt CEs under alternative bending conditions were studied. Flexible CEs with continuous and non-continuous Pt films were prepared by sputtering and electroless deposition, respectively. Both electrodes were used in this study to understand the influence of Pt structure on the bending behavior of the flexible Pt CEs. It was demonstrated that the electrocatalytic activity of the continuous Pt CE slightly increases with the increasing bending cycles while the non-continuous Pt CE reveals much higher resistance towards bending tests.
2. Experimental section
2.1 Preparation of flexible Pt CEs
The continuous and non-continuous Pt CEs were prepared by sputtering and electroless deposition, respectively, according to the processes described in our previous study.25,26 For the sputtered Pt CE, continuous Pt film was deposited on ITO-PEN substrate (PECF-IP, 15 Ω sq−1, Peccell) using a magnetron sputtering system (Explorer 14, Denton Vacuum, 7 min) at room temperature. For the electroless-deposited (ED) Pt CE, the cleaned ITO-PEN plastic substrate was first immersed into a sensitized solution containing 0.03 mM SnCl2 and 160 mM HCl at 25 °C for 3 min to absorb Sn2+ ions onto the ITO surface. After rinsing with distilled water it was then soaked into an activating solution (1.3 mM H2PtCl6 and 120 mM HCl) at 25 °C for 2 min to deposit Pt seeds on the ITO surface through the reduction of PtCl62− by Sn2+. The activated ITO-PEN film was then dipped in a plating solution (2.4 mM H2PtCl6, 60 mM HCl and 1.5 mM hydrazine hydrate) at 60 °C for 0.5 min to prepare non-continuous Pt film. Finally, the Pt-deposited ITO-PEN film was rinsed with distilled water at 60 °C, and then dried at ambient conditions.2.2 Physical and electrochemical characterization of Pt CEs
The surface morphology of the flexible Pt CEs was characterized by a field emission scanning electron microscope (FESEM, QUANTA 600F). The Pt loading of the flexible Pt CEs was measured using an inductively coupled plasma-atomic emission spectrometer (ICP-AES, IRIS Advantage).The electrocatalytic activity of the flexible Pt CEs was investigated by electrochemical impedance spectroscopy (EIS) using a blank cell including two identical Pt CEs, which were separated by a 60 μm-thick Surlyn film (1702, DuPont). The cell with an active area of 0.9 cm2 was filled with an electrolyte composed of 0.6 M DMPII (Institute of Plasma Physics, China), 0.05 M I2 (Aldrich), 0.1 M LiI (Aldrich), and 0.5 M 4-tert-butylpyridine (Acros) in anhydrous acetonitrile (Aldrich). The EIS spectra were measured by a potentiostat (Solartron 1287) equipped with a frequency response analyzer (Solartron 1260) at 0 V and 10 mV amplitude over a frequency range of 10−1 to 105 Hz in the dark. Impedance parameters were determined through fitting the EIS spectra by Z-view software with an equivalent circuit.4
2.3 Bending tests of Pt CEs
The Pt blank cells made in Section 2.2 were chosen for the bending tests in order to measure the in situ change of the electrocatalytic activity. Fig. 1 shows the schematic structures of the blank cell and the real solar cell. As can be seen, the neutral layer is nearly in the same position for the both cells, suggesting that the stress of the Pt CE in the blank cell and solar cell under bending state is same. Therefore the results from bending tests for blank cells can represent the cases in real DSC devices.The bending test was carried out by a home-developed flexible solar cell bending tester, by which the bending conditions, including bending direction, bending radius and bending cycle, were accurately and automatically controlled.1,2 Fig. S1 (ESI†) shows a bending cycle which includes alternating forward and backward stress applications. Moreover, the electrocatalytic activities of the flexible Pt CEs after multiple bending, measured by EIS, were used to assess the bending behavior of the flexible Pt CEs.
3. Results and discussion
3.1 Surface morphologies of the flexible Pt CEs
Fig. 2 shows the surface morphologies of the flexible Pt CEs. It can be seen that the sputtered Pt CE (Fig. 2b) exhibits a similar surface morphology to the bare ITO-PEN substrate (Fig. 2a), suggesting that a uniform and continuous Pt layer is formed on the ITO surface by the deposition of Pt atoms in vapor phase. In addition, the Pt loading amount of the sputtered Pt CE is 232 μg cm−2, giving a thickness of the Pt layer at ca. 108 nm. As seen from Fig. 2c, the surface of the activated ITO-PEN substrate is slightly etched, and a few irregular blocks are clearly observed. Our previous study shows that this etching effect seems to be critical for the successful electroless deposition of Pt film on the ITO-PEN substrate,25 possibly due to the effective surface modification by etching a thin layer of the ITO-PEN substrate. Fig. 2d shows the image for the ED-Pt CE with a deposition duration of 0.5 min. The Pt nanoparticles are uniformly distributed on the activated ITO-PEN surface, resulting from the growth of the initial Pt nuclei on the activated ITO surface through a self-catalytic pathway.27 It is evident that the ED-Pt CE presents a non-continuous Pt distribution, which is much different from the continuous Pt distribution on the sputtered Pt CE.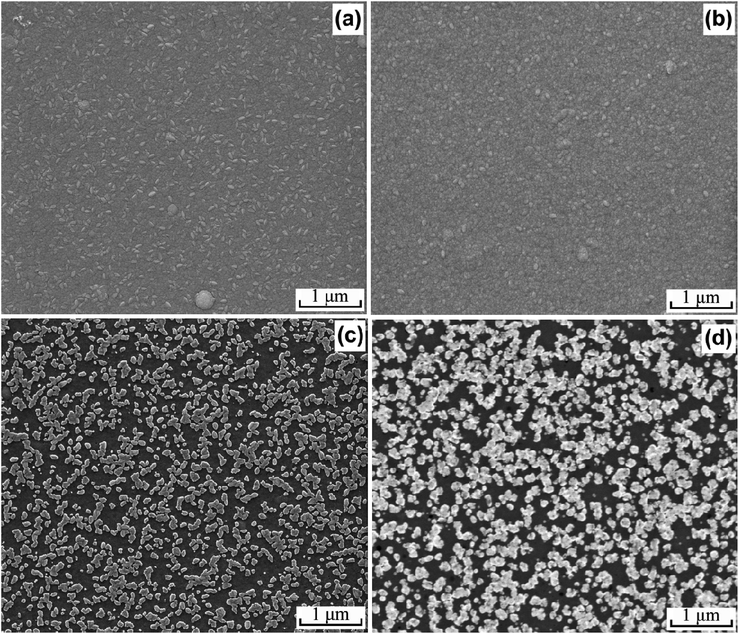 | ||
| Fig. 2 Surface morphologies of the bare ITO-PEN film (a), sputtered Pt CE (b), activated ITO-PEN film (c), and ED-Pt CE (d). | ||
3.2 Electrocatalytic activity of the flexible Pt CEs
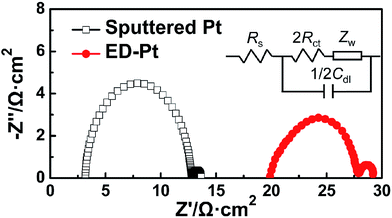
The electrocatalytic activity of the Pt CEs was investigated by EIS. Fig. 3 shows the typical Nyquist plots of the flexible Pt CEs. By using the equivalent circuit in the inset of Fig. 3, the series resistance (Rs) and the charge transfer resistance at the Pt/electrolyte interface (Rct), two parameters for evaluating the electrocatalytic activity of the Pt CE, can be obtained. The Rct values are 4.8 and 3.2 Ω cm2 for the sputtered Pt CE and ED-Pt CE, respectively.It should be noted that, the electrocatalytic activity of the Pt CE will change with the standing duration.28 Before the bending test, the influence of standing duration on the electrocatalytic activity of the flexible Pt CE was also studied, as shown in Fig. S2 (ESI†). The relative Rct, calculated from the ratio of the measured Rct and the original Rct, decreases and thereby the electrocatalytic activity improves with increasing the standing duration. After standing for 4 h, the Rct value of the Pt CE decreases by less than 10%. In this study, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of bending takes 1 h. Therefore, the change of electrocatalytic activity should be taken into account in the calculation during the subsequently bending test.
000 cycles of bending takes 1 h. Therefore, the change of electrocatalytic activity should be taken into account in the calculation during the subsequently bending test.
3.3 Bending behavior of the continuous Pt CE
Fig. 4 shows the influence of bending radius and bending cycle on the relative series resistance Rs for the continuous Pt CE. It is obvious that the Rs value remains almost unchanged by changing bending radius up to 18 mm and bending cycle to 1000. The variations of the relative Rct of the continuous Pt CE with the various bending radius and bending cycles are shown Fig. 4b and c. Considering the influence of standing duration on the electrocatalytic activity of the Pt CE as described previously (i.e., in Section 3.2), when the bending radius is greater than 12 mm, the relative Rct value of the Pt CE decreases and thereby the electrocatalytic activity slightly improves with the increased bending cycle. Interestingly, it was found that when the bending radius is 8 mm, the relative Rct value firstly decreases, and then increases with the increased bending cycle. Besides, when the bending cycles are the same, the relative Rct value of the Pt CE decreases and thereby the electrocatalytic activity slightly improves with the decrease of the bending radius.It is well known that the electrocatalytic activity is determined by the effective surface area of the Pt film.5 To have a further insight into the mechanism for the improved electrocatalytic activity of the continuous Pt CE after repeated bending, the surface morphologies of the Pt CEs were examined by SEM analyses. Fig. 5 shows the surface morphologies of the continuous Pt CE after different bending conditions. It is clearly observed that there are delamination and cracking on the Pt film (Fig. 5b and c), which will increase the bare effective surface area of the Pt film and thereby increase the electrocatalytic activity of the Pt CE. However, when the bending radius is 8 mm, as can be seen from Fig. 5d, some of the Pt film spalls off from the ITO surface, resulting in decreased effective surface area and therefore decreased electrocatalytic activity of the Pt CE. The spall off of the Pt CE is detrimental to the solar cell, which should be definitely avoided in fabricating the flexible DSCs.
Based on the results shown above, a possible failure model for the continuous Pt CE after repeated bending is proposed and shown in Fig. 6. The Pt film is firstly delaminated after repeated bending, and then cracks with the increase of bending cycle. This will directly increase the bare effective surface area of the Pt film and thereby increase the electrocatalytic activity of the Pt CE. In addition, the local cracks on the surface of the Pt CE do not significantly affect the electric conductivity of the Pt CE. Furthermore, some of the Pt film will spall off from the ITO surface, leading to the possible potential failure of the flexible DSCs.
3.4 Bending behavior of the non-continuous Pt CE
Fig. 7a shows the influence of bending radius and bending cycle on the relative Rs values of discontinuous Pt CE. It is obvious that the Rs values keep unchanged by changing bending radius and bending cycle. The variations of the relative Rct values of non-continuous Pt CE with the bending radius and bending cycle are shown in Fig. 7b. Taking the influence of standing duration on the electrocatlytic activity of Pt CE (Fig. S2 (ESI†)) into account, when the bending radius is 18 mm, the relative Rct values of the Pt CE keep unchanged with the increasing bending cycles. Moreover, the relative Rct value of the Pt CE shows a similar dependency on bending cycle when the bending radius is 12 mm and 8 mm. Therefore, the electrocatalytic activity of the non-continuous Pt CE is nearly the same even with bending cycles up to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
 | ||
| Fig. 7 Influence of bending radius and bending cycle on the relative Rs (a) and Rct (b) values of non-continuous Pt CE. | ||
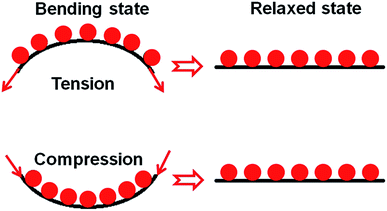
To have a better understanding on the unchanged electrocatalytic activity of the non-continuous Pt CE after repeated bending, the surface morphologies of the Pt CE were observed. Fig. 8 shows the surface morphologies of the Pt CEs after different bending conditions. It is clearly seen that all the morphologies are nearly the same and consist of the dispersed Pt nanoparticles. In addition, Pt nanoparticles may spall off from the ITO surface. However, the change of Pt loading is difficult to evaluate the spall off of Pt nanoparticles. Therefore, the coverage ratio, defined by the ratio of the Pt nanoparticles area on the ITO surface to the whole ITO surface area, of the Pt nanoparticles on the ITO surface after different bending conditions is calculated by counting at least 10 surface morphology images of the Pt CE. The statistic results are shown in Fig. 8e, from which it is clear that the coverage ratio of the Pt nanoparticles is unchanged after repeated bending. In brief, the electrocatalytic activity of the discontinuous Pt CE is nearly the same after repeated bending. This is mainly attributed to the discontinuous structure along the in-plane direction (i.e. the load direction under bending condition in this study) of the discontinuous Pt CE, which can help the release of stress during bending service as illustrated in Fig. 9. This is somewhat similar to the Au nanomeshes on elastic substrate,29 but different from the system reported by Lu et al. in which a metal film was developed on a plastic substrate without a brittle ceramic layer.30 In contrast, the continuous Pt layer on the continuous Pt CE is completely integrated with the substrate, and thereby bears the larger stress resulting from the bending of the Pt CE.
 | ||
| Fig. 8 Surface morphologies (a–d) and the variation of Pt coverage ratio (e) of non-continuous Pt CEs after different bending conditions. | ||
Compared with the continuous Pt film, the non-continuous Pt film is relatively rough in the surface, which may have some effect on the performance of the Pt film. Fan et al.14 reported an interesting result when they compared a hierarchical nanostructured carbon material, hollow core-mesoporous shell carbon with a commercial activated carbon material with a larger surface roughness factor, suggesting that a larger surface roughness factor (i.e., a ratio of the real surface area to the geometrical area of a porous material) may contribute something to the improved electrode performance, however, the contribution from the surface roughness factor is generally smaller compared with that from the surface nanostructure.
In addition, the photovoltaic performance of the flexible DSCs before and after Pt CE bending has been attempted in this study. However, bending Pt CE and then fabricating DSCs may bring some unpredictable effects to the photovoltaic performance of the solar cells, therefore bending solar cells was chosen in this study. Unfortunately, our recent results show that the TiO2 film may crack or spall off during bending service,31 therefore it is difficult to exclude the effect of TiO2 film. Despite this, the findings in this study on bending Pt CEs can provide knowledge of their contribution to the whole DSC performance under bending services.
4. Conclusions
In summary, the behaviors of flexible Pt CEs with continuous and non-continuous films, which were prepared by sputtering and electroless deposition, respectively, under alternating bending were studied. It was found that the electrocatalytic activity of the continuous Pt CE slightly increases with the increase in the bending cycle, due to the delamination and cracking of the Pt film. A bending failure model for the continuous Pt CE is also proposed. In contrast, the electrocatalytic activity of the non-continuous Pt CE keeps unchanged with the increasing bending cycle. This is mainly attributed to the high strain tolerance and unique surface structure of the non-continuous Pt CE, which would be beneficial to the release of stress during bending service.Acknowledgements
This work was supported by the National Science Foundation of China (No. 51072160), National Program for Support of Top-notch Young Professionals.References
- X. He, M. Liu, G. Yang, H. Yao, S. Fan and C. Li, J. Power Sources, 2013, 226, 173–178 CrossRef CAS PubMed.
- X. He, G. Yang, C. Li, C. Li and S. Fan, J. Power Sources, 2014, 251, 122–129 CrossRef CAS PubMed.
- X. He, G. Yang, C. Li and C. Li, Appl. Surf. Sci., 2014, 288, 416–422 CrossRef CAS PubMed.
- A. Hauch and A. Georg, Electrochim. Acta, 2001, 46, 3457–3466 CrossRef CAS.
- N. Papageorgiou, Coord. Chem. Rev., 2004, 248, 1421–1446 CrossRef CAS PubMed.
- M. Grätzel, Nature, 2001, 414, 338–344 CrossRef PubMed.
- M. S. Dresselhaus and I. L. Thomas, Nature, 2001, 414, 332–337 CrossRef CAS PubMed.
- S.-Q. Fan, B. Fang, J. Kim, B. Jeong, C. Kim, J. Yu and J. Ko, Langmuir, 2010, 26, 13644–13649 CrossRef CAS PubMed.
- S. Ahmad, J. Yum, X. Zhang, M. Grätzel, H. Butta and M. K. Nazeeruddin, J. Mater. Chem., 2010, 20, 1654–1658 RSC.
- D. E. Bartak, B. Kazee, K. Shimazu and T. Kuwana, Anal. Chem., 1986, 58, 2756–2761 CrossRef CAS.
- J. Kim, B. Fang, M. Kim and J.-S. Yu, Catal. Today, 2009, 146, 25–30 CrossRef CAS PubMed.
- M.-S. Kim, B. Fang, N. Chaudhari, M. Song, T. Bae and J.-S. Yu, Electrochim. Acta, 2010, 55, 4543–4550 CrossRef CAS PubMed.
- P. Millet, F. Andolfatto and R. Durand, Int. J. Hydrogen Energy, 1996, 21, 87–93 CrossRef CAS.
- S.-Q. Fan, B. Fang, J. Kim, J. Kim, J. Yu and J. Ko, Appl. Phys. Lett., 2010, 96, 063501 CrossRef PubMed.
- H. Nishikiori, R. A. Setiawan, K. Miyashita, K. Teshima and T. Fujii, Catal. Sci. Technol., 2013, 3, 1512–1519 CAS.
- T. Yamaguchi, N. Tobe, D. Matsumoto, T. Nagai and H. Arakawa, Sol. Energy Mater. Sol. Cells, 2010, 94, 812–816 CrossRef CAS PubMed.
- T. Ma, X. Fang, M. Akiyama, K. Inoue, H. Noma and E. Abe, J. Electroanal. Chem., 2004, 574, 77–83 CrossRef CAS PubMed.
- X. Fang, T. Ma, M. Akiyama, G. Guan, S. Tsunematsu and E. Abe, Thin Solid Films, 2005, 472, 242–245 CrossRef CAS PubMed.
- K. Lee, S. Wu, C. Chen, C. Wu, M. Ikegami, K. Miyoshi, T. Miyasaka and K. Ho, J. Mater. Chem., 2009, 19, 5009–5015 RSC.
- L. Lin, C. Lee, R. Vittal and K. Ho, J. Power Sources, 2010, 195, 4344–4349 CrossRef CAS PubMed.
- S. Kim, Y. Nah, Y. Noh, J. Jo and D. Kim, Electrochim. Acta, 2006, 51, 3814–3819 CrossRef CAS PubMed.
- S. Ito, N. L. C. Ha, G. Rothenberger, P. Liska, P. Comte, S. M. Zakeeruddin, P. Pechy, M. K. Nazeeruddin and M. Gratzel, Chem. Commun., 2006, 4004–4006 RSC.
- L. Chen, W. Tan, J. Zhang, X. Zhou, X. Zhang and Y. Lin, Electrochim. Acta, 2010, 55, 3721–3726 CrossRef CAS PubMed.
- L. Yang, L. Wu, M. Wu, G. Xin, H. Lin and T. Ma, Electrochem. Commun., 2010, 12, 1000–1003 CrossRef CAS PubMed.
- X. He, M. Liu, G. Yang, S. Fan and C. Li, Appl. Surf. Sci., 2011, 258, 1377–1384 CrossRef CAS PubMed.
- X. He, M. Liu, G. Yang, S. Fan, C. Li and C. Li, Rare Met. Mater. Eng., 2012, 41, 464–466 Search PubMed.
- Z. Zhou, W. Zhou, S. Wang, G. Wang, L. Jiang, H. Li, G. Sun and Q. Xin, Catal. Today, 2004, 93–95, 523–528 CrossRef CAS PubMed.
- G. Syrrokostas, A. Siokou, G. Leftheriotis and P. Yianoulis, Sol. Energy Mater. Sol. Cells, 2012, 103, 119–127 CrossRef CAS PubMed.
- C.-F. Guo, T. Sun, Q. Liu, Z. Suo and Z. Ren, Nat. Commun., 2014, 5, 3121 Search PubMed.
- N. Lu, X. Wang, Z. Suo and J. Vlassak, Appl. Phys. Lett., 2007, 91, 221909–221911 CrossRef PubMed.
- X. He, G. Yang, C. Li, M. Liu and S. Fan, J. Power Sources, 2015, 280, 182–189 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details on schematic of flexible Pt CE undergoes a bending cycle of alternating bending mode, and relative Rct value of the Pt CE shown against the standing duration. See DOI: 10.1039/c5ra13256h |
| This journal is © The Royal Society of Chemistry 2015 |