Conversion of straw to nitrogen doped carbon for efficient oxygen reduction catalysts in microbial fuel cells†
Abstract
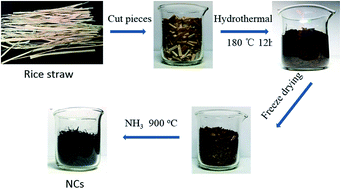
The exploration of highly-efficient and low-cost oxygen reduction reaction (ORR) catalysts was still a major task for microbial fuel cells (MFCs). Herein, we report the conversion of cheap and widely available biomass to nitrogen-doped carbon (NC) for highly-efficient ORR catalysts in air-cathode MFCs. The NC was prepared from rice straw through a three-step process, including hydrothermal carbonization, freeze drying and heat-treatment in NH3. The NC had a high nitrogen content of 5.57 at% and showed outstanding catalytic activity for ORR. The MFC based on the NC generated a high maximum power density of 2300 mW m−2, which was much higher than that of the Pt/C.

 Please wait while we load your content...
Please wait while we load your content...