A simple strategy to enhance electrical conductivity of nanotube-conjugate polymer composites via iodine-doping†
Abstract
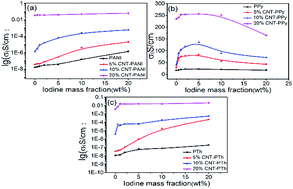
Iodine-doping is useful in the development of high-conductivity carbon nanotube (CNT)–polymer composites. We prepared three types of CNT–polymer composites by in situ polymerization and obtained iodine-doped samples by mechanical mixing. A series of studies on electrical conductivity has proven that iodine doping is an effective way to obtain high-conductivity composites with heteroatoms in the polymer matrix. The conductivities of the doped samples increased by 4 orders of magnitude compared to the undoped samples. Based on the Hall-effect, Raman and XPS spectra, we propose that the synergistic effect between CNT and iodine results in superior properties. When the heteroatom is N, the synergistic effect of iodine and CNT helps to form a stronger p–π conjugated system. The N cation radicals (as a type of carrier) increase with the enhanced conjugation, resulting in the improved conductivity. When the heteroatom is S, CNT and iodine form a separate π–π conjugated system and charge-transfer complex with S. The combination of the two interactions induces a boost in the carrier concentration, as well as the conductivity.

 Please wait while we load your content...
Please wait while we load your content...