Fabrication of paper-based microfluidic analysis devices: a review
Abstract
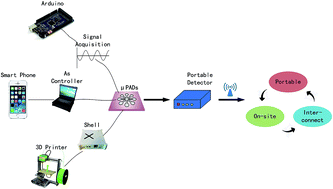
When compared with conventional microfluidic chips made of glass and polymer substrates, paper-based microfluidic analysis devices (μPADs) possess many unique advantages, including low-cost, easy-to-fabricate, strong capillary action and good biological compatibility. In recent years, μPADs have attracted increased interest and attention, which has led to their rapid development. Thousands of literature reports regarding μPADs have been published and a variety of μPADs fabrication methods have been reported. This review focuses on the development of the fabrication methods of 2D and 3D μPADs since 2007. A summary of the advantages and disadvantages of these methods is provided with particular attention paid to the resolution and cost of each method. Suitable applications of each method are discussed. Also, some trends of μPADs are summarized.

 Please wait while we load your content...
Please wait while we load your content...