Transcriptional regulation of xylose utilization in Enterococcus mundtii QU 25†
Hiroaki Yanasea,
Tomoko Araya-Kojimaa,
Yuh Shiwa‡
b,
Satoru Watanabea,
Takeshi Zendoc,
Taku Chibazakuraa,
Mariko Shimizu-Kadotada,
Kenji Sonomotoc and
Hirofumi Yoshikawa*ab
aDepartment of Bioscience, Tokyo University of Agriculture, 1-1-1 Sakuragaoka, Setagaya-ku, Tokyo 156-8502, Japan
bGenome Research Center, NODAI Research Institute, Tokyo University of Agriculture, 1-1-1 Sakuragaoka, Setagaya-ku, Tokyo 156-8502, Japan. E-mail: hiyoshik@nodai.ac.jp; Fax: +81-3-5477-2668; Tel: +81-3-5477-2758
cLaboratory of Microbial Technology, Division of Systems Bioengineering, Department of Bioscience and Biotechnology, Faculty of Agriculture, Graduate School, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan
dDepartment of Environmental Sciences, Musashino University, 3-3-3 Ariake, Koto-ku, Tokyo 135-8181, Japan
First published on 26th October 2015
Abstract
Enterococcus mundtii QU 25, a non-dairy lactic acid bacterium, produces optically pure L-lactic acid (≥99.9%) via homo-fermentation when cultured in the presence of xylose at high concentrations. However, as the xylose concentration decreases, a metabolic shift to hetero-lactic fermentation occurs in this strain. Furthermore, this strain preferentially metabolizes glucose when cultured in medium containing high concentrations of both glucose and xylose, indicating that a previously uncharacterized carbon-catabolite repression system may govern the regulation of these processes. Therefore, to increase the productivity of pure L-lactate by QU 25, it is necessary to investigate this regulatory process. In this study, we performed transcriptional analyses, including RNA sequencing to analyze the transcriptome of QU 25 cultivated in the presence of various glucose and/or xylose concentrations. Our results demonstrate that there was a gradual reduction in the expression of several genes in the xylose gene cluster as the glucose concentration increased, and that there was robust transcription of the genes involved in hetero-lactic fermentation under homo-lactic fermentation conditions. The former result indicates that transcriptional regulation of genes in the xylose gene cluster is involved in the catabolite repression observed in QU 25. The latter results show that the metabolic shift between homo- and hetero-lactic fermentation in QU 25 is not caused by the transcriptional regulation of related genes under the conditions tested. We therefore propose that a yet uncharacterized transcriptional regulation process is involved in the observed catabolite repression.
1. Introduction
Xylose is a major component of renewable plant-derived biomass. This aldopentose is typically found in hemicellulose, which is present along with cellulose and lignin in biomass. Currently, efforts have been focused on expanding the types of biomass used as feedstock for bioconversion. After saccharification of plant-derived biomass, nonselective utilization of xylose and glucose is highly desired. Green plastic poly-L-(+)-lactic acid is comprised of optically pure L-lactic acid, which can be obtained only by microbial fermentation. Furthermore, L-lactic acid has a number of valuable commercial uses, including as a food preservative.1Enterococcus mundtii QU 25 is a non-dairy lactic acid bacterium (LAB), isolated from ovine feces, that ferments three aldopentoses (arabinose, ribose, and xylose).2 This strain produces high levels of L-lactic acid when cultured under optimal conditions using glucose or xylose as the sole carbon source, and its use for bioconversion of plant-derived biomass has been proposed.3,4 Recently, we analyzed and annotated the complete 3.02 Mb genome sequence of QU 25,5 and demonstrated that this organism encodes the genes necessary for xylose utilization and homo- and hetero-lactic fermentation (Table 1).
| Feature ID | Gene name | Chromosomal region | Strand direction | GC (%) | Predicted function | |
|---|---|---|---|---|---|---|
| Start | End | |||||
| a Aliases are described in parenthesis. | ||||||
| Genes concerned in xylose fermentation and carbon catabolite repression (CCR) (examined in Tables 2 and 3) | ||||||
| Xylose gene cluster | ||||||
| EMQU_2811 | xylR | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 917 917![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 694 694 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 918 918![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 845 845 |
+ | 40.54 | Xylose repressor |
| EMQU_2810 | xylA | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 916 916![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127 127 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 917 917![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434 434 |
− | 41.13 | Xylose isomerase |
| EMQU_2809 | xynB | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 914 914![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 407 407 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 916 916![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 023 023 |
− | 42.86 | Xylan β-1,4-xylosidase |
| EMQU_2808 | araG | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 912 912![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 855 855 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 914 914![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 366 366 |
− | 40.41 | Ribose transport system ATP-binding protein |
| EMQU_2805 | xylB | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 909 909![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 196 196 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 910![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 680 |
− | 44.18 | D-Xylulose kinase |
| Pentose-phosphate/glycolytic pathway | ||||||
| EMQU_1275 | tktA1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 334 334![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 628 628 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 336 336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622 622 |
+ | 45.61 | Transketolase |
| EMQU_2812 | tktA3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 918 918![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 972 972 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920 920![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 966 966 |
− | 46.22 | Transketolase |
| EMQU_2814 | talC | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 921 921![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 874 874 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 922 922![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 527 527 |
− | 44.19 | Transaldolase |
| Phosphoketolase pathway | ||||||
| EMQU_1837 | xfpA (ptk)a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 913 913![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 814 814 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 916 916![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 189 189 |
− | 43.73 | D-Xylulose 5-phosphate/D-fructose 6-phosphate phosphoketolase |
| EMQU_2620 | ackA | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 690 690![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 711 711 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 691 691![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892 892 |
− | 37.23 | Acetate kinase |
| EMQU_2119 | eutD (pta)a | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 204 204![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 462 462 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205 205![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 442 442 |
− | 39.45 | Phosphotransacetylase |
| EMQU_1120 | pflB | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 175 175![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 401 401 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 177 177![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 632 632 |
+ | 40.37 | Formate acetyltransferase |
| EMQU_0224 | adhE | 231![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590 590 |
234![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 187 187 |
+ | 40.99 | Bifunctional acetaldehyde-CoA/alcohol dehydrogenase |
| CCR proteins | ||||||
| EMQU_0954 | ptsH | 989![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353 353 |
989![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 619 619 |
+ | 38.95 | PTS system transporter protein HPr |
| EMQU_1943 | ccpA | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 032 032![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 634 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 033 033![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 635 635 |
− | 38.42 | catabolite control protein A |
| EMQU_1951 | hptK | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 039 039![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 941 941 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 879 879 |
− | 42.07 | HPr kinase/phosphorylase |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Aldopentose transporter genes (examined in Table 4) | ||||||
| D-Xylose transport system proteins | ||||||
| EMQU_1845 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 925![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107 107 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926 926![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198 198 |
− | 39.93 | D-Xylose transport system substrate-binding protein | |
| EMQU_1844 | araG | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 923![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924 924![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 812 812 |
− | 40.77 | D-Xylose transport system ATP-binding protein |
| EMQU_1843 | araH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 922 922![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 078 078 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 923![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280 |
− | 42.39 | D-Xylose transport system permease protein |
| Ribose transport system proteins (included in xylose gene cluster) | ||||||
| EMQU_2808 | araG | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 912 912![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 855 855 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 914 914![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 366 366 |
− | 40.41 | Ribose transport system ATP-binding protein |
| EMQU_2807 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 911 911![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 848 848 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 912 912![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 813 813 |
− | 39.03 | Ribose transport system permease protein | |
| EMQU_2806 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 910![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 773 773 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 911 911![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 822 822 |
− | 41.52 | Ribose transport system substrate-binding protein | |
| Other ribose transport system proteins | ||||||
| EMQU_1846 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926 926![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425 425 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 927 927![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 390 |
+ | 39.65 | Ribose transport system substrate-binding protein | |
| EMQU_2382 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 453 453![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523 523 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 453 453![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 918 918 |
− | 38.64 | Ribose transport protein RbsD | |
| EMQU_2381 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 452 452![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 618 618 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 453 453![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 505 505 |
− | 41.78 | Ribose uptake protein | |
| L-Arabinose transport system proteins | ||||||
| EMQU_1582 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 640 640![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343 343 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 641 641![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 632 632 |
− | 41.01 | Lactose/L-arabinose transport system substrate-binding protein | |
| EMQU_1581 | lacF2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 639 639![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 118 118 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 639 639![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 987 987 |
− | 35.29 | Lactose/L-arabinose transport system permease protein |
| EMQU_1580 | lacG2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638 638![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 243 243 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 639 639![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121 121 |
− | 37.77 | Lactose/L-arabinose transport system permease protein |
In a previous study, when QU 25 was cultured in medium containing 50 g L−1 glucose and 50 g L−1 xylose, both sugars were utilized simultaneously. Notably, however, while glucose was preferred over xylose, this strain did not exhibit a diauxic two-step growth curve. Specifically, the maximum glucose consumption rate of QU 25 was 5.72 g L−1 h−1, while that of xylose was 1.17 g L−1 h−1, and only 42.9% of the xylose was metabolized.6 This phenomenon cannot be explained by the well-characterized mechanism of carbon catabolite repression (CCR) exhibited by low G + C count Gram-positive bacteria (Firmicutes). In CCR, genes encoding transporters and enzymes involved in the metabolism of a particular sugar(s) are repressed in the presence of a preferred sugar such as glucose. Subsequent exhaustion of the preferred sugar, however, triggers transcription of the repressed genes followed by uptake and metabolism of the second sugar. This metabolic process results in a canonical diauxic growth curve (reviewed in ref. 7). While it is possible that QU 25 employs a type of CCR when cultured in the presence of glucose, we previously demonstrated that this strain consumed 98.0% of the xylose with a similar consumption rate as glucose when cultured in medium containing 25 g L−1 glucose and 50 g L−1 xylose, which is inconsistent with this hypothesis.6
In a previous study, QU 25 produced optically pure (≥99.9%) L-lactic acid via homo-fermentation when cultured in media containing xylose as the sole carbon source (100 g L−1), and it was predicted that this process involved the pentose-phosphate (PP)/glycolytic pathway.4 While no phosphoketolase (PK) activity, which comprises the first step in the hetero-lactic fermentative PK pathway, was detected at high xylose concentrations,4 a metabolic shift to hetero-lactic fermentation was observed at concentrations less than 25 g L−1, which was accompanied by the formation of byproducts, such as acetic acid, formic acid, and ethanol, as well as PK activity, and the activity of enzymes involved in the PP/glycolytic pathway.4
To increase the yield of pure L-lactate produced by QU 25 from biomass, it is crucial to investigate the CCR-like phenomenon that governs xylose metabolism and the metabolic shift to hetero-lactic fermentation employed by this strain. In this study, we utilized RNA sequencing (RNA-seq) to analyze the transcriptome of QU 25 in the presence of varying concentrations of glucose and xylose, as well as northern hybridization analysis to assess the expression of genes involved in xylose fermentation, and primer extension analysis of the xylose gene cluster to determine the transcriptional start site (TSS) of the xylose isomerase gene (xylA). Using these approaches, we demonstrate that transcriptional regulation is involved in the CCR observed in QU 25. However, the metabolic shift of QU 25 was not mediated by the transcriptional regulation of xylose metabolism-related genes under the conditions tested.
2. Results
2.1 RNA-seq analysis of QU 25 cultured under low sugar conditions
A previous study demonstrated that CCR of xylose utilization genes does not occur in the QU 25 strain when cultured in media containing xylose and glucose if the glucose concentration is below 25 g L−1.6 In addition, this strain exhibits hetero-lactic fermentation in the presence of low concentrations (≤25 g L−1) of xylose as a sole carbon source.4 Therefore, to examine the transcriptome of this strain when cultured in the presence of low sugar concentrations, we performed RNA-seq analysis on QU 25 cultured in G5, X5, and G1X5 media (see Experimental 4.1). The results of this analysis are summarized in Table 2 (genes involved in xylose metabolism and CCR) and ESI Table S2† (all genes). Growth curves for cells cultured under these conditions are depicted in ESI Fig. S1.† The results demonstrate that QU 25 grew more slowly in the X5 medium than in all other media evaluated.| Gene name | RPKMa (G5) | RPKM (X5) | RPKM (G1X5) | X5/G5b | G1X5/G5b |
|---|---|---|---|---|---|
| a RPKM stands for reads per kb of exon per million mapped reads.b X5/G5 and G1X5/G5 indicate the RPKM (X5)/RPKM (G5) and RPKM (G1X5)/RPKM (G5) values, respectively. | |||||
| Xylose gene cluster | |||||
| xylR | 122.39 | 214.78 | 195.41 | 1.75 | 1.60 |
| xylA | 856.04 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 096.79 096.79 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 383.75 383.75 |
18.80 | 15.63 |
| xynB | 594.43 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 023.59 023.59 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473.84 473.84 |
16.86 | 20.98 |
| araG | 323.81 | 6110.76 | 9164.44 | 18.87 | 28.30 |
| xylB | 282.55 | 3059.00 | 2270.88 | 10.83 | 8.04 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Pentose-phosphate/glycolytic pathway | |||||
| tktA1 | 120.15 | 201.08 | 116.81 | 1.67 | 0.97 |
| tktA3 | 37.04 | 11.68 | 12.40 | 0.32 | 0.33 |
| talC | 218.56 | 208.95 | 160.38 | 0.96 | 0.73 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Phosphoketolase pathway | |||||
| xfpA | 158.58 | 421.98 | 196.78 | 2.66 | 1.24 |
| ackA | 920.55 | 595.13 | 483.45 | 0.65 | 0.53 |
| eutD | 716.65 | 585.16 | 895.70 | 0.82 | 1.25 |
| pflB | 4501.62 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 246.12 246.12 |
6833.48 | 2.50 | 1.52 |
| adhE | 1089.53 | 8384.88 | 3772.75 | 7.70 | 3.46 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| CCR proteins | |||||
| ptsH | 7039.75 | 4088.60 | 6796.87 | 0.58 | 0.97 |
| ccpA | 209.62 | 329.06 | 167.39 | 1.57 | 0.80 |
| hptK | 504.83 | 327.44 | 535.85 | 0.65 | 1.06 |
2.2 Northern hybridization analysis of the xylose gene cluster and identification of the xylA TSS under low sugar conditions
To confirm the results of our RNA-seq analysis, we performed northern hybridization to examine the expression levels of the genes in the xylose gene cluster under identical culturing conditions.As shown in Fig. 1A, the xylA probe detected three mRNA molecules expressed by QU 25 that were approximately 1.3, 6.4, and 10 kilobases (kb) in size, when cultured in X5 and G1X5 media. However, this probe did not detect any transcripts in the G5 medium, indicating that the transcripts detected were induced by xylose and not repressed by glucose in the medium containing both sugars. These results were therefore consistent with the gene expression patterns obtained by our RNA-seq analysis. The 10 kb mRNA molecule that was detected by the xylA probe was also detected in the QU 25 RNA samples by the xynB, araG, and xylB probes after culturing in the X5 and G1X5 media. Likewise, the 6.4 kb mRNA was detected by the xylA, xynB, and araG probes, but not by the xylB probe, in these samples. Given that the xylA coding sequence is 1308 base pairs (bp) in length (see Table 1), the two larger mRNA molecules were likely polycistronic mRNAs, while the 1.3 kb mRNA contained only the xylA sequence. In addition to the 10 kb band, the xylB probe detected a 5.7 kb mRNA in the samples from cells cultured in the X5 and G1X5 media, which was larger than the xylB gene (1485 bp in length; Table 1). Therefore, xylB seems to also be transcribed as another operon that does not include xylA, xynB, or araG. Lastly, the xylR probe detected a single 1.1 kb mRNA in the samples obtained from all three cultures. As the xylR gene is 1152 bp in length (see Table 1), this finding is consistent with the monocistronic transcription of this gene. Furthermore, the transcriptional level of xylR in the G5 medium was less than that observed in the other two xylose-containing media, which also corresponds to the results of the RNA-seq analysis.
Using primer extension analysis, we determined that the TSS of the xylA gene is a thymidine (T) that is 63 bp upstream of the xylA translational start codon (Fig. 1B and C). Within the whole genome sequence, this thymidine is base 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 917
917![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497. As such, the 10 kb transcript detected by northern hybridization is estimated to start at this position and to terminate near the end of the EMQU_2802 (base 2
497. As such, the 10 kb transcript detected by northern hybridization is estimated to start at this position and to terminate near the end of the EMQU_2802 (base 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 906
906![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 796) locus, a region encompassing the xynB, araG, EMQU_2807, EMQU_2806, xylB, EMQU_2804, and EMQU_2803 genes (Fig. 2). Similarly, the 6.4 kb mRNA is predicted to comprise the sequences encoded at the locus between this TSS and near the end of the EMQU_2806 (base 2
796) locus, a region encompassing the xynB, araG, EMQU_2807, EMQU_2806, xylB, EMQU_2804, and EMQU_2803 genes (Fig. 2). Similarly, the 6.4 kb mRNA is predicted to comprise the sequences encoded at the locus between this TSS and near the end of the EMQU_2806 (base 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910
910![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 773), which includes the xynB, araG, and EMQU_2807 genes. The predicted promoter region of xylA is indicated by double bars in Fig. 1C. Based on the results of the northern hybridization and primer extension analyses, we deduced the structure of the operon encoding the xylose gene cluster (Fig. 2).
773), which includes the xynB, araG, and EMQU_2807 genes. The predicted promoter region of xylA is indicated by double bars in Fig. 1C. Based on the results of the northern hybridization and primer extension analyses, we deduced the structure of the operon encoding the xylose gene cluster (Fig. 2).
2.3 RNA-seq analysis of QU 25 cultured under high sugar conditions
In previous analyses, QU 25 exhibited CCR when cultivated under high sugar conditions (i.e. consumed glucose faster than xylose and left a portion of the xylose unused), such as in medium containing a 50 g L−1 glucose and 50 g L−1 xylose mixture.6 QU 25 also exhibited homo-lactic fermentation when cultured with 100 g L−1 of xylose as the sole carbon source.4 Therefore, to examine the gene expression profile of QU 25 under high sugar conditions, we performed a second RNA-seq analysis of cells cultured in the following media: G150, X150, G10X50, G25X50, G50X50, and G100X50 (see Experimental 4.1). Table 3 summarizes the data regarding the genes involved in xylose metabolism and CCR while ESI Table S3† contains the data for all genes. The growth curves of cells under the described conditions are shown in ESI Fig. S2.† The results show that QU 25 grew more slowly in the X150 medium, compared to all other media evaluated.| Gene name | RPKMa (G10X50) | RPKM (G25X50) | RPKM (G50X50) | RPKM (G100X50) | RPKM (G150) | RPKM (X150) | G10X50/G150b | G25X50/G150b | G50X50/G150b | G100X50/G150b | X150/G150b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a RPKM stands for reads per kb of exon per million mapped reads.b G10X50/G150, G25X50/G150, G50X50/G150, G100X50/G150, and X150/G150 indicate the RPKM (G10X50)/RPKM (G150), RPKM (G25X50)/RPKM (G150), RPKM (G50X50)/RPKM (G150), RPKM (G100X50)/RPKM (G150), and RPKM (X150)/RPKM (G150) values, respectively. | |||||||||||
| Xylose gene cluster | |||||||||||
| xylR | 285.70 | 306.97 | 286.65 | 330.83 | 102.60 | 387.34 | 2.78 | 2.99 | 2.79 | 3.22 | 3.78 |
| xylA | 4645.56 | 2364.84 | 1828.10 | 828.19 | 22.27 | 6503.77 | 208.62 | 106.20 | 82.09 | 37.19 | 292.06 |
| xynB | 2888.53 | 1581.22 | 1264.01 | 420.03 | 19.65 | 3328.79 | 146.99 | 80.47 | 64.32 | 21.38 | 169.40 |
| araG | 1837.77 | 881.84 | 704.14 | 147.71 | 17.14 | 2265.47 | 107.22 | 51.45 | 41.08 | 8.62 | 132.17 |
| xylB | 1851.55 | 868.18 | 595.27 | 113.08 | 41.31 | 1930.46 | 44.82 | 21.01 | 14.41 | 2.74 | 46.73 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Pentose-phosphate/glycolytic pathway | |||||||||||
| tktA1 | 364.42 | 490.99 | 462.83 | 333.90 | 191.32 | 507.90 | 1.90 | 2.57 | 2.42 | 1.75 | 2.65 |
| tktA3 | 7.36 | 5.50 | 4.49 | 4.70 | 2.63 | 13.33 | 2.80 | 2.09 | 1.71 | 1.79 | 5.08 |
| talC | 31.94 | 14.78 | 20.72 | 13.77 | 23.35 | 81.77 | 1.37 | 0.63 | 0.89 | 0.59 | 3.50 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Phosphoketolase pathway | |||||||||||
| xfpA | 110.50 | 93.15 | 137.87 | 147.80 | 135.94 | 256.22 | 0.81 | 0.69 | 1.01 | 1.09 | 1.88 |
| ackA | 973.39 | 1150.10 | 922.43 | 1129.22 | 954.51 | 1463.87 | 1.02 | 1.20 | 0.97 | 1.18 | 1.53 |
| eutD | 1487.04 | 1435.64 | 1242.74 | 1210.44 | 1170.24 | 1676.58 | 1.27 | 1.23 | 1.06 | 1.03 | 1.43 |
| pflB | 3903.36 | 3811.22 | 2596.54 | 1900.55 | 1211.73 | 4982.09 | 3.22 | 3.15 | 2.14 | 1.57 | 4.11 |
| adhE | 4204.20 | 1986.76 | 2483.65 | 2388.84 | 1577.48 | 2582.87 | 2.67 | 1.26 | 1.57 | 1.51 | 1.64 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| CCR proteins | |||||||||||
| ptsH | 9658.17 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533.67 533.67 |
9079.38 | 9383.88 | 7610.33 | 6777.32 | 1.27 | 1.38 | 1.19 | 1.23 | 0.89 |
| ccpA | 631.45 | 829.44 | 800.99 | 737.95 | 506.26 | 577.99 | 1.25 | 1.64 | 1.58 | 1.46 | 1.14 |
| hptK | 390.59 | 356.97 | 418.84 | 412.58 | 371.45 | 374.78 | 1.05 | 0.96 | 1.13 | 1.11 | 1.01 |
However, a gradual reduction in the transcriptional expression levels of xylA, xynB, and araG occurred in a glucose concentration-dependent manner. Indeed, while the G10X50/G150 values for these genes ranged from 107.22 to 208.62, these values decreased to 51.45–106.20 (G25X50/G150), 41.08–82.09 (G50X50/G150), and 8.62–37.19 (G100X50/G150) as the glucose concentration increased. Meanwhile, the RPKM values for the xylB gene were nearly identical in the G10X50 and X150 media (1851.55 and 1930.46, respectively); however, as the glucose concentration increased, the relative values for this gene exhibited a reduction similar to that observed with the xylA, xynB, and araG genes. Specifically, the G10X50/G150, G25X50/G150, G50X50/G150, and G100X50/G150 values were 44.82, 21.01, 14.41, and 2.74, respectively. It is therefore conceivable that glucose or a glucose metabolite negatively regulates transcription of the xylose gene cluster, resulting in the CCR effect observed in QU 25. Notably, the transcriptional levels of the xylR gene, which was annotated as a repressor, were nearly constant regardless of the glucose concentration of the medium (G10X50/G150, G25X50/G150, G50X50/G150, and G100X50/G150 = 2.78, 2.99, 2.79, and 3.22, respectively).
Similar to the genes of the PP/glycolytic pathway, the highest expression levels of the PK pathway genes xfpA and ack, which are involved in hetero-lactic fermentation, were observed in the X150 medium (RPKM = 256.22 and 1463.87, respectively), and the transcript levels of these genes were not affected by changes in the glucose concentration. Conversely, while eutD and pflB also exhibited the highest transcript levels in the X150 medium (RPKM = 1676.58 and 4982.09, respectively), the expression of these genes decreased in a manner similar to that of the xylose gene cluster, albeit more moderately, as the glucose concentration in the medium increased. The highest expression level of the adhE gene was observed in the G10X50 medium (RPKM = 4204.20), and the relative RPKM values in the other xylose-containing media (compared to that in the G150 medium) were between 1.26 and 2.67. It was noteworthy that multiple genes, including xfpA, demonstrated such robust expression in the X150 medium despite the fact that QU 25 exhibited homo-lactic fermentation. Furthermore, no phosphoketolase activity was previously detected in the presence of high (100 g L−1) xylose concentration.4
| Feature ID | RPKMa (G10X50) | RPKM (G25X50) | RPKM (G50X50) | RPKM (G100X50) | RPKM (G150) | RPKM (X150) | G10X50/X150b | G25X50/X150b | G50X50/X150b | G100X50/X150b | G150/X150b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a RPKM stands for reads per kb of exon per million mapped reads.b G10X50/X150, G25X50/X150, G50X50/X150, G100X50/X150, and G150/X150 indicate the RPKM (G10X50)/RPKM (X150), RPKM (G25X50)/RPKM (X150), RPKM (G50X50)/RPKM (X150), RPKM (G100X50)/RPKM (X150), and RPKM (G150)/RPKM (X150) values, respectively. | |||||||||||
| D-Xylose transport system proteins | |||||||||||
| EMQU_1845 | 46.11 | 3.50 | 7.23 | 5.43 | 8.25 | 107.76 | 0.43 | 0.03 | 0.07 | 0.05 | 0.08 |
| EMQU_1844 | 20.10 | 4.01 | 7.38 | 6.86 | 8.20 | 70.03 | 0.29 | 0.06 | 0.11 | 0.10 | 0.12 |
| EMQU_1843 | 17.96 | 4.85 | 10.99 | 8.41 | 12.83 | 72.50 | 0.25 | 0.07 | 0.15 | 0.12 | 0.18 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Ribose transport system proteins (included in xylose gene cluster) | |||||||||||
| EMQU_2808 | 1837.77 | 881.84 | 704.14 | 147.71 | 17.14 | 2265.47 | 0.81 | 0.39 | 0.31 | 0.07 | 0.01 |
| EMQU_2807 | 1589.56 | 576.10 | 490.50 | 76.12 | 17.79 | 1882.41 | 0.84 | 0.31 | 0.26 | 0.04 | 0.01 |
| EMQU_2806 | 2555.99 | 993.96 | 750.61 | 95.35 | 22.27 | 2921.43 | 0.87 | 0.34 | 0.26 | 0.03 | 0.01 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Other ribose transport system proteins | |||||||||||
| EMQU_1846 | 31.26 | 20.64 | 21.16 | 23.61 | 20.06 | 47.03 | 0.66 | 0.44 | 0.45 | 0.50 | 0.43 |
| EMQU_2382 | 107.58 | 81.37 | 81.75 | 84.64 | 261.49 | 224.77 | 0.48 | 0.36 | 0.36 | 0.38 | 1.16 |
| EMQU_2381 | 109.57 | 73.36 | 83.26 | 93.44 | 252.13 | 212.93 | 0.51 | 0.34 | 0.39 | 0.44 | 1.18 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| L-Arabinose transport system proteins | |||||||||||
| EMQU_1582 | 7.78 | 4.21 | 7.16 | 6.98 | 12.10 | 31.92 | 0.24 | 0.13 | 0.22 | 0.22 | 0.38 |
| EMQU_1581 | 4.99 | 2.89 | 5.67 | 5.81 | 6.35 | 16.04 | 0.31 | 0.18 | 0.35 | 0.36 | 0.40 |
| EMQU_1580 | 5.41 | 5.04 | 6.50 | 8.46 | 9.17 | 20.64 | 0.26 | 0.24 | 0.31 | 0.41 | 0.44 |
2.4 Northern hybridization analysis of xylA under high sugar conditions
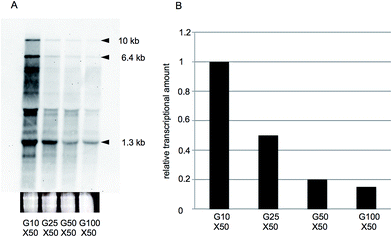
To confirm these results, we again performed northern hybridization analysis of xylA gene expression using RNA samples harvested from cells cultivated in G10X50, G25X50, G50X50, and G100X50 media. As observed under low sugar conditions, the xylA probe detected three mRNA species (1.3, 6.4, and 10 kb in size, respectively) after culturing in the high xylose-containing media (Fig. 3A), and there was a gradual reduction in the densities of these bands as the glucose concentration in the medium increased (Fig. 3B). These results are therefore consistent with the RNA-seq data summarized in Tables 3 and S3† for all genes, indicating the reproducibility and accuracy of our results.3. Discussion
In this study, we utilized RNA-seq analysis to examine the gene expression profile of E. mundtii QU 25, which is an efficient L-lactic acid-producing LAB in the presence of varying glucose and xylose concentrations. Furthermore, we performed northern hybridization analysis to assess the expression of genes involved in the xylose gene cluster as well as their transcriptional activity. The results of the northern analysis (Fig. 1 and 3) were consistent with those of the RNA-seq analyses (Tables S2 and S3† for all genes), indicating the reproducibility and accuracy of our results. Moreover, our results, particularly those of the RNA-seq analysis of QU 25 cultured under high sugar conditions, indicate that (i) the CCR observed previously in QU 25 (ref. 6) was controlled at the transcriptional level, while (ii) the metabolic shift between homo- and hetero-lactic fermentation, according to the initial concentration of xylose as a sole carbon source in the media, was not under transcriptional control under the conditions tested in this study.Fujita categorized CCR in Firmicutes into two groups: CcpA-dependent and CcpA-independent CCR.7 The former involves the CcpA complex including the seryl-phosphorylated form of HPr (P-Ser-HPr), which is a negative repressor. This complex then binds to specific sites (cre sites) near promoter regions that function as operators to regulate transcriptional expression. In this typical CCR, the bacterium exhibits a diauxic two-step growth curve. However, CcpA-independent CCR mechanisms involve either the transcriptional repressors CggR or CcpN, or the histidyl-phosphorylated form of HPr (P-His-HPr), which is involved in the phosphotransferase transport system (PTS) (reviewed in ref. 7). However, cggR and ccpN are not present in the QU 25 genome, indicating that this CCR mechanism may not occur in this organism.
Therefore, we examined the possibility that CcpA-dependent CCR may regulate xylose and glucose utilization in QU 25. We identified a putative cre sequence (ATGAAAGCGTATACTA) in the xylA promoter region, indicated by a dashed line in Fig. 1C, that exhibits 92% identity (excluding Ns) with the consensus cre sequence (WTG3NNARC8G9NWWWC14AW, where W stands for A or T, R for A or G, and N for any base).9 Notably, however, G3, C8, G9, and C14 are the only bases that are completely conserved within experimentally verified cre sites.9 The sequence identified in this study also contains these four conserved bases, which supports the notion that this region is a cre site. Furthermore, the 3′-end of this sequence included the −10 region of the predicted xylA promoter. It is therefore possible that CcpA is involved in the CCR-mediated regulation of xylose utilization by QU 25. In a previous study, however, Zomer et al. (2007) reported several genes that contained a putative cre site but did not exhibit observable CCR regulation.10 Therefore, it is also conceivable that the xylA gene in QU 25 might be an example of such a gene.
Next, we examined the phenomenon reported by Asanuma et al. (2004) that the transcriptional expression level of ccpA was higher in media containing a more rapidly utilizable energy source in Streptococcus bovis that exhibits CcpA-dependent CCR.11 In contrast to these findings, there were similar expression levels of ccpA in QU 25 cultured under low or high sugar conditions (X5/G5 and X150/G150 = 1.57 and 1.14, respectively; Tables 2 and 3). Moreover, QU 25 grew faster in media containing glucose only than in media containing xylose (ESI Fig. S1 and S2†). Therefore, the putative involvement of CcpA in the CCR of QU 25 was unclear.
It was evident from our findings that the genes involved in xylose transport and metabolism were transcribed in media containing a mixture of glucose and xylose. However, the transcript levels of these genes decreased as the glucose concentration of the medium increased. These observations, as well as the simultaneous consumption of glucose and xylose by QU 25, are not consistent with a model of hierarchical metabolism of these sugars via a CcpA-dependent CCR pathway. Therefore, we propose the existence of a CcpA-independent CCR mechanism in QU 25 that regulates the simultaneous metabolism of xylose and glucose. There is precedence for this phenomenon, as Mortera et al. (2012) reported the coexistence of both CcpA-dependent and CcpA-independent CCR mechanisms in Enterococcus faecalis.12
Notably, QU 25 harbors more genes that encode transporters related to lactic acid fermentation, including both ABC transporters and a PTS, than other enterococcal species or even the other E. mundtii strain.13 Xylose is a member of the aldopentose group, which includes arabinose, ribose, and xylose. In QU 25, the aldopentose transporter genes were highly repressed under high glucose conditions (Table 4). Furthermore, the ribose ABC transporter genes in the xylose gene cluster (EMQU_2806–2808) were highly transcribed in the presence of xylose but were gradually repressed as the glucose concentration increased, indicating that these proteins could participate in the CCR of QU 25. That these genes exhibited the lowest G150/X150 values among the transporter genes examined (Table 4) strengthens this supposition. Conversely, no PTS genes specific to aldopentose transport were identified in QU 25. We therefore predict that the ribose ABC transporter genes in the xylose gene cluster contribute to the CCR observed in QU 25 under high glucose conditions, and we summarize a model for the xylose fermentation pathway of QU 25 in Fig. 4.
 | ||
| Fig. 4 Map of the xylose fermentation pathway in Enterococcus mundtii QU 25. The genes that exhibited glucose-dependent variations in transcriptional expression in media containing high sugar concentrations are indicated with bold type (see Tables 2 and 3). Thick arrowheads denote that there was a marked decrease in the transcriptional expression levels of the corresponding genes under high glucose conditions. Conversely, for genes where there was minimal change or fluctuation in expression under these conditions, the arrow was omitted. Abbreviations in this figure are as follows: P, phosphate; GAP, glyceraldehyde 3-phosphate. Dotted lines indicate the phosphoketolase (PK) pathway, which exerts only in the hetero-fermentation conditions. Solid lines indicate the other pathways including the pentose-phosphate (PP)/glycolytic pathway, which exert both in the homo- and hetero-fermentation conditions. | ||
We propose that the metabolic shift between homo- and hetero-lactic fermentation in the presence of xylose as a sole carbon source is regulated in QU 25 downstream of the transcriptional level. This conclusion is supported by our findings that transcription of the genes involved in the PK pathway was maintained under homo-fermentation conditions, indicating that the shift to hetero-lactic fermentation is not under transcriptional control; however, the mechanism governing this switch remains unclear. For efficient production of optically pure lactic acid, homo-lactic fermentation of pentoses and hexoses is highly desired. In the case of pentoses, such as xylose, up to five moles of lactic acid are synthesized from three moles of xylose via the homo-fermentative PP/glycolytic pathway. As such, the theoretical yield of lactic acid produced per xylose consumed is 1.67 mol mol−1. In a previous study, the maximal yield of L-lactic acid produced by QU 25 was 1.51 mol mol−1 when cultured with 50 g L−1 xylose.4 Considering that sugars are used to increase cell mass rather than to produce lactate during exponential growth of bacteria, this is a remarkable yield and strengthens the importance of QU 25 in the green plastic industry.
The role of the XylR repressor cannot be ignored when investigating the transcriptional regulation of the xylose gene cluster. Like other known xylose gene clusters that are repressed by XylR in the absence of xylose,14 the expression of the genes within the xylose gene cluster of QU 25 was also repressed in the absence of xylose and induced in its presence. The putative XylR-binding site, GTTAGTTTGTTAGATAAACTAAC, which is indicated by a dotted line in Fig. 1C, exhibited 95% identity (excluding Ns) to the consensus sequence GTTWGTTTATNNNATAAACWAAC among all Firmicutes, except Lactobacillus pentosus, Lactobacillus brevis, Lactococcus lactis, and Clostridium acetobutylicum.15 However, the position of the first base in this site in QU 25 was at +7, which is markedly further downstream than that of other XylR regulons in Firmicutes.15,16 Dahl et al. (1995) reported that XylR contributes to glucose repression because glucose 6-phosphate is an anti-inducer that prevents xylose-mediated induction in Bacillus subtilis, B. megaterium, and B. licheniformis.17 Accordingly, this possibility should also be considered for QU 25.
4. Experimental
4.1 Bacterial strain and culturing conditions
E. mundtii QU 25, isolated from ovine feces,2 was cultured in M17-2 medium (M17 medium lacking yeast extract and lactose)18 supplemented with various concentrations of glucose and/or xylose at 37 °C. Media were named according to the type and concentration of sugars used: 5 g L−1 glucose (G5); 150 g L−1 glucose (G150); 5 g L−1 xylose (X5); 150 g L−1 xylose (X150); 1 g L−1 glucose and 5 g L−1 xylose (G1X5); 10 g L−1 glucose and 50 g L−1 xylose (G10X50); 25 g L−1 glucose and 50 g L−1 xylose (G25X50); 50 g L−1 glucose and 50 g L−1 xylose (G50X50); and 100 g L−1 glucose and 50 g L−1 xylose (G100X50).4.2 RNA preparation
Logarithmic phase cultures (OD600 of 0.8) were utilized for RNA extraction. Cultures (8 mL) were collected by centrifugation and frozen at −80 °C. Total RNA was then extracted using the QIAGEN RNeasy Mini Kit (Qiagen, Venlo, Netherlands).4.3 RNA-seq
The total RNA from each culture was prepared for analysis as follows: rRNA was removed from each sample using the Ribo-Zero™ Magnetic Kit for Gram-positive bacteria (Epicentre [an Illumina company], Madison, WI, USA) and cDNA libraries were generated using the NEBNext mRNA Library Prep Reagent Set for Illumina (New England BioLabs, Ipswich, MA, USA). Libraries prepared from cultures under low sugar conditions (Table 2) were then analyzed using the GAIIx Next-Generation Sequencing (NGS) platform (Illumina, San Diego, CA, USA) by 50-base-paired-end sequencing. Meanwhile, libraries prepared from cultures under high sugar conditions (Table 3) were analyzed using the HiSeq 2000 system (Illumina) by 50-base-single-end sequencing. Each sequence was mapped to the QU 25 genome (deposited in GenBank/DDBJ/EMBL under accession numbers AP013036–AP013041) using the CLC Genomics Workbench software version 7.0.4 (length fraction = 0.5, similarity fraction = 0.8, maximum number of hits for a read = 1) (CLC bio, Aarhus, Denmark). Mapped reads of each gene were normalized using the RPKM approach.194.4 Northern hybridization analysis
Chemically labeled RNA probes were prepared using the DIG RNA Labeling Kit (SP6/T7) (Roche, Basel, Switzerland) by in vitro transcription using T7 RNA polymerase of the cloned region into the pSPT18 vector. Oligonucleotide primers used for PCR amplification of the corresponding DNA fragments for cloning into the vector were synthesized by Hokkaido System Science Co., Ltd. (Sapporo, Japan; shown in ESI Table S1†). RNA (10 μg) was denatured by incubation at 65 °C for 10 min and then subjected to agarose gel electrophoresis on a 1% agarose gel containing 2.22% formaldehyde and 1× 3-(N-morpholino)propanesulfonic acid (MOPS) buffer (20 mM MOPS, 1 mM ethylenediaminetetraacetic acid [EDTA], and 5 mM sodium acetate [pH 7.0]). Perfect RNA Markers (0.2–10 kb; Novagen [a brand of Merck KGaA], Darmstadt, Germany) was used as a size marker. The RNA was then transferred to Hybond N+ membranes (GE Healthcare, Little Chalfont, United Kingdom) by capillary action using 20× SSC (1× SSC = 0.15 M NaCl and 0.015 M sodium citrate) as transfer buffer and by placing a weight on the gel-contacted membrane and incubating at room temperature (r.t.) overnight. Hybridization was performed at 60 °C overnight using DIG Easy Hyb Granules (Roche), followed by washing twice at r.t. for 5 min with 2× SSC–0.1% SDS solution. This was then followed by another two washes at 60 °C for 15 min with 0.5× SSC–0.1% SDS solution. An anti-digoxigenin–AP conjugate (Roche) treatment was then performed and the membrane was washed twice at r.t. for 15 min with 2× SSC–0.1% SDS solution. The density of each band was quantified using Image Lab™ software (version 2.0; Bio-Rad, Hercules, CA, USA).4.5 Primer extension method for identification of the xylA TSS
RNA was extracted from 8 mL of cells cultured in M17-2 medium supplemented with 5 g L−1 xylose. Primer extension analysis was performed using BcaBEST™ RNA PCR Kit Ver.1.1 (Takara Bio Inc., Otsu, Japan) by annealing 10 pmol of IRDye800-labeled primer (MS TechnoSystems, Osaka, Japan) to 15 μg of RNA. This was followed by incubation at 58 °C for 1 h. The GATC sequence ladder was produced using the DNA fragment that was amplified by PCR using two oligonucleotide primers (ESI Table S1†), the same primer described above, and Ex Taq DNA polymerase (Takara Bio). Following an initial 5 min denaturation at 95 °C, 30-cycle amplifications were performed with denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 70 °C for 1 min. A 6% Long Ranger running gel (Long Ranger™ Gel Solutions [Lonza], Basel, Switzerland) was used for analysis.4.6 RNA-seq data accession numbers
The RNA-seq data obtained in this study were deposited in the DNA Data Bank of Japan Sequence Read Archive (DRA)/National Center for Biotechnology Information Sequence Read Archive (SRA)/European Bioinformatics Institute Sequence Read Archive (ERA) under the accession number DRA002964.5. Conclusion
In this paper, we examined the atypical CCR mechanism employed by the QU 25 strain under high glucose conditions, and provided evidence that the transcriptional regulation of the genes in the xylose gene cluster played a role in this CCR process. We propose that this regulation pathway involves a CcpA-independent mechanism. Furthermore, we speculate that the ribose ABC transport system encoded in the xylose gene cluster may contribute to this process. We also demonstrated that the observed metabolic shift between homo- and hetero-lactic fermentation in QU 25 is regulated downstream of the transcriptional expression of the enzymes involved in xylose metabolism.Acknowledgements
We thank Shiori Sonobe for performing the northern hybridization analysis, Kiyotaka Abe for generating the growth curves under high sugar conditions, and Yu Kanesaki for submission of the RNA-seq data to DDBJ. This study was supported by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2013–2017 (S1311017).References
- M. A. Abdel-Rahman, Y. Tashiro and K. Sonomoto, J. Biotechnol., 2011, 156, 286–301 CrossRef CAS.
- M. A. Abdel-Rahman, Y. Tashiro, T. Zendo, K. Shibata and K. Sonomoto, Appl. Microbiol. Biotechnol., 2011, 89, 1039–1049 CrossRef CAS PubMed.
- M. A. Abdel-Rahman, Y. Tashiro, T. Zendo and K. Sonomoto, RSC Adv., 2013, 3, 8437–8445 RSC.
- M. A. Abdel-Rahman, Y. Tashiro, T. Zendo, K. Hanada, K. Shibata and K. Sonomoto, Appl. Environ. Microbiol., 2011, 77, 1892–1895 CrossRef CAS PubMed.
- Y. Shiwa, H. Yanase, Y. Hirose, S. Satomi, T. Araya-Kojima, S. Watanabe, T. Zendo, T. Chibazakura, M. Shimizu-Kadota, H. Yoshikawa and K. Sonomoto, DNA Res., 2014, 21, 369–377 CrossRef CAS PubMed.
- M. A. Abdel-Rahman, Y. Xiao, Y. Tashiro, Y. Wang, T. Zendo, K. Sakai and K. Sonomoto, J. Biosci. Bioeng., 2015, 119, 153–158 CrossRef CAS PubMed.
- Y. Fujita, Biosci., Biotechnol., Biochem., 2009, 73, 245–259 CrossRef CAS PubMed.
- F. Titgemeyer, J. Reizer, A. Reizer and M. H. Sailer Jr, Microbiology, 1994, 140, 2349–2354 CrossRef CAS PubMed.
- M. A. Schumacher, M. Sprehe, M. Bartholomae, W. Hillen and R. G. Brennan, Nucleic Acids Res., 2011, 39, 2931–2942 CrossRef CAS.
- A. L. Zomer, G. Buist, R. Larsen, J. Kok and O. P. Kuipers, J. Bacteriol., 2007, 189, 1366–1381 CrossRef CAS PubMed.
- N. Asanuma, T. Yoshii and T. Hino, Appl. Environ. Microbiol., 2004, 70, 5244–5251 CrossRef CAS PubMed.
- P. Mortera, M. Espariz, C. Suárez, G. Repizo, J. Deutscher, S. Alarcón, V. Blancato and C. Magni, Appl. Environ. Microbiol., 2012, 78, 1936–1945 CrossRef CAS PubMed.
- Y. Shiwa, Ph.D. thesis, Tokyo University of Agriculture, 2014.
- D. Gärtner, J. Degenkolb, J. A. E. Ripperger, R. Allmansberger and W. Hillen, Mol. Gen. Genet., 1992, 232, 415–422 CrossRef.
- D. A. Rodionov, A. A. Mironov and M. S. Gelfand, FEMS Microbiol. Lett., 2001, 205, 305–314 CrossRef CAS PubMed.
- Y. Gu, Y. Ding, C. Ren, Z. Sun, D. A. Rodionov, W. Zhang, S. Yang, C. Yang and W. Jiang, BMC Genomics, 2010, 11, 255 CrossRef PubMed.
- M. K. Dahl, D. Schmiedel and W. Hillen, J. Bacteriol., 1995, 177, 5467–5472 CAS.
- M. Machii, S. Watanabe, T. Zendo, T. Chibazakura, K. Sonomoto, M. Shimizu-Kadota and H. Yoshikawa, J. Biosci. Bioeng., 2013, 115, 481–484 CrossRef CAS PubMed.
- A. Mortazavi, B. A. Williams, K. McCue, L. Schaeffer and B. Wold, Nat. Methods, 2008, 5, 621–628 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: The RNA-seq data obtained in this study were deposited in the DNA Data Bank of Japan Sequence Read Archive (DRA)/National Center for Biotechnology Information Sequence Read Archive (SRA)/European Bioinformatics Institute Sequence Read Archive (ERA) under the accession number DRA002964. See DOI: 10.1039/c5ra15028k |
| ‡ Present address: Iwate Tohoku Medical Megabank Organization, Iwate Medical University, 2-1-1 Nishitokuta, Yahaba-cho, Shiwa-gun, Iwate 028-3694, Japan. |
| This journal is © The Royal Society of Chemistry 2015 |