Co3O4@Highly ordered macroporous carbon derived from a mollusc shell for supercapacitors
Yahui Liu,
Wei Yu,
Li Hou,
Guanhong He and
Zhihong Zhu*
Institute of Nano-science and Nano-technology, College of Physical Science and Technology, Central China Normal University, Wuhan, 430079, China. E-mail: zhzhu@phy.ccnu.edu.cn
First published on 28th August 2015
Abstract
Bio-derived honeycomb-like ordered macroporous carbon with highly interconnected hexangular channels and excellent electronic conductivity is prepared by carbonizing the organic matrix of mollusc shells. The unique structure ensures fast electron transfer throughout the entire three-dimensional network and effective electrolyte penetration, which makes the mollusc shell derived carbon network (MSDCN) an ideal substrate for supercapacitor electrodes. Via a simple hydrothermal process, cubic Co3O4 was incorporated with the MSDCN, and the as-obtained Co3O4@MSDCN exhibits an outstanding electrochemical performance with high specific capacitance (1307 F g−1 at 1 A g−1), impressive rate performance (with a capacity retention of 61.0% at 20 A g−1) and long cycle life (84% of the initial capacitance remained after 3000 cycles at 5 A g−1).
1. Introduction
Energy storage has become a great challenge worldwide as the quantity of fossil-fuel energy is rapidly decreasing.1 Supercapacitors have been in the spotlight;2 they have many potential practical applications, such as energy storage devices, on account of their excellent features of comparatively high specific power density, fast charge–discharge rate and long cycle life,3,4 and outstanding stability at extreme temperatures.5 Based on the working principles, supercapacitors are classified as electric double-layer capacitors (EDLCs) and pseudocapacitors.6,7 Unlike EDLCs, which store charges electrostatically by reversibly adsorbing electrolyte ions onto electrodes, pseudocapacitors store charges through fast and reversible surface or near-surface faradic reactions,6 always leading to a much higher specific performance.7–9The electrode material principally determines the energy storage properties of pseudocapacitors.10 For decades, various types of transition metal oxide electrode materials have been studied, such as CoO,11,12 MnO2,13 RuO2,14,15 NiO16 and so forth. Among these metal oxides, cobalt oxide has well-defined redox activity, great reversibility, controllable size and shape and high theoretical specific capacitance.17,18 Cobalt oxide, which has a theoretical specific capacitance of 3560 F g−1,19–21 is generally considered to be an ideal electrode candidate material for supercapacitors because of its favourable capacitive characteristics and environmental friendliness.19,22,23 Nonetheless, for Co3O4 as well as the other transition metal oxide electrode materials, they are always subjected to the deficiency of electrical and ionic conductivity; thus, how to supply electrons and ions with efficient delivery and diffusion is under rigorous consideration with regard to the process of synthesizing electrode materials.11,24 To satisfy this urgent requirement, growing Co3O4 on a carbon-based material that has good electrical conductivity and a high surface area to obtain the composites can be an alternative method to enable rapid energy storage and release.25,26 Numerous available carbon materials, such as CNTs and graphene, have been applied to electrode fabrication.27 Nevertheless, these materials are costly and difficult to synthesize. The most obvious defect of CNTs and graphene is their discontinuous structure, which notably decreases the electron delivery, ion diffusion and impedes their range of application as substrates.24,28
In recent years, large quantities of low-cost bio-derived carbon materials have been used as carbon substrates in many approaches. For example, Lan et al. derived a rose-based carbon skeleton to scaffold CoO nanocubes.11 Wu et al. synthesized the composites of carbon gel from watermelon and Fe3O4 nanoparticles, which exhibited an excellent specific capacitance of 333.1 F g−1.29 Yu et al. obtained composites of wood-derived carbon and PANI for supercapacitor electrode materials.30 In general, it is feasible to apply bio-derived materials to high-performance supercapacitors as both a substrate and active material.
In this work, a type of renewable bio-carbon substrate, the mollusc shell derived carbon network (MSDCN), was originally obtained through an acid treatment and a simple argon atmosphere protected carbonization process. Obviously, the costless and eco-friendly fabrication process of MSDCN is advantageous for its commercial application. Furthermore, it has an inherent fine structure. MSDCN with hierarchical hexagonal channel arrays has outstanding geometric continuity, ensuring the effective utilization of space of the carbon matrix and facilitating electron transfer so as to promote the conductivity of the composites. In addition, the regular channels with large volumes increase electrolyte infiltration for higher ion diffusion rate of the electrode. To implement its potential in energy storage, we directly grow cubic Co3O4 on the MSDCN. The uniformly aligned cubic Co3O4 offers sufficient contact between the active materials and electrolyte to significantly facilitate the efficiency. As a result, Co3O4@MSDCN composites exhibit remarkable electrochemical performance with a high specific capacitance of 1307 F g−1 at 1 A g−1 and as high as 798 F g−1 at 20 A g−1. After 3000 cycles, 84% of the capacitance is maintained at 5 A g−1. The experimental results illustrate that the obtained MSDCN is potentially applicable to the fabrication of supercapacitor electrodes.
2. Experimental
2.1 Preparation of mollusc shell derived carbon network (MSDCN)
By applying an improved method from a previous study to our work,24 the MSDCN was prepared. First, the mollusc shell was cleaned and polished with emery paper until the periostracum was removed. Then, to eliminate calcium carbonate, the mollusc shell was immersed in a 6 M HCl solution for 24 h at room temperature. After completely decalcification, the nacreous layers and prismatic layers could be easily stripped. Eventually, the prismatic layers were dried in a lyophilizer and carbonized at 900 °C in an argon flow (85 sccm) for 2 h. As a result, the ideal MSDCN was obtained.2.2 Synthesis of macroporous Co3O4@MSDCN
All of the solvents and chemicals used were of analytical grade without further purification. In brief, 0.4 g of Co(NO3)·6H2O and 0.2 g of CTAB were dissolved in 22.8 mL of deionized water with 6 mL of methanol under vigorous magnetic stirring for 30 min to become homogeneous at room temperature. Then, the solution was transferred into an 80 mL Teflon-lined stainless steel autoclave. A piece of the as-prepared MSDCN was subsequently immersed into the reaction solution. The autoclave was sealed, maintained at 180 °C for 15 h and subsequently let it naturally cool to room temperature. Then, the product was removed from the solution and washed with deionized water and ethanol several times. After it was dried in air at 60 °C for 5 h, the product was converted to Co3O4 at 250 °C in air for 4 h with a heating rate of 1 °C per minute to obtain the Co3O4@MSDCN composites.2.3 Characterizations
The crystalloid structure of the product was characterized using X-ray diffraction (XRD, X'Pert PRO MRD, PANalytical, Netherlands) and Raman Spectroscopy (LabRAMHR evolution, 532 nm). The morphologies of the product were observed using field-emission scanning electron microscopy (SEM; JEOL, JSM-6700F) and high-resolution transmission electron microscopy (TEM; JEM-2100(HR), 200 kV).2.4 Electrochemical measurements
The electrochemical measurements were performed in a three-electrode electrochemical cell, which consists of the active material as the working electrode, a platinum rod as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode; a 6 M KOH aqueous solution was used as the electrolyte. The cubic-Co3O4 electrode was prepared according as follows. Briefly, 80 wt% Co3O4 powder, 10 wt% acetylene black as a conducting agent and 10 wt% poly-vinylidene fluoride (PVDF) as a binder were homogeneously mixed and pressed onto nickel foam, which served as the current collector. The area of the Co3O4@MSDCN composites is 1 × 1.5 cm2 and the mass loading of Co3O4 on MSDCN is 1.6 mg. The electrochemical behaviour of the cubic-Co3O4 electrode was characterized using a series of cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) tests and EIS tests. These tests were performed on a PARSTAT 4000 electrochemical workstation. The CV measurements were performed over a potential range of −0.2 to 0.6 V at various scanning rates. The GCD measurements were performed in a potential window of 0 to 0.37 V. The cycling performance was performed on Arbin SCTS.The specific capacitance was calculated according to the following equation:
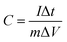 | (1) |
3. Results and discussion
The fabrication of Co3O4@MSDCN composites consists of the following steps, as shown in Fig. 1. First, the organic matrix (prismatic layer) was peeled from the mollusc shell after total decalcification. Then, the organic matrix was lyophilized and annealed to obtain the macroporous interconnected carbon network. Finally, Co3O4 was incorporated with MSDCN using a simple hydrothermal method.To characterize the morphology, scanning electron microscopy (SEM) was applied. Fig. 2a displays the macroporous interconnected structure of the organic matrix of a mollusc shell. After annealing, the organic matrix was carbonized, as shown in Fig. 2b. Compared with the organic matrix before carbonization, the MSDCN exhibits a more regular porous structure without fractures. The average diameter of the pores is approximately 25 μm. There are no cracks and defects between adjacent pores, which illustrates that the MSDCN has a superior mechanical property and exactly satisfies the requirement of supercapacitor composites to load active materials. Therefore, this macroporous interconnected carbon network, which is an inexpensive and environmentally friendly potential composite in energy storage, will certainly exhibit high performance after it is compounded with Co3O4.
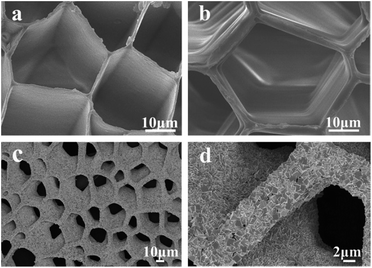 | ||
| Fig. 2 SEM images of (a) the structure of the macroporous interconnected network, (b) MSDCN, (c) and (d) composites of the macroporous Co3O4@MSDCN at different magnifications. | ||
To grow Co3O4 on MSDCN, a hydrothermal process was performed after the MSDCN was oxidized via acid treatment which advances the interfacial adhesion strength between Co3O4 and MSDCN surface.24,31 The chemical reaction is described as follows:32,33
| Co2+ + 2OH− → (α)-Co(OH)2 → (β)-Co(OH)2 | (2) |
Subsequently, the hydroxide transforms to the stable oxide phase in air.
| Co(OH)2 + O2 → Co3O4 + H2O | (3) |
The hydroxyl ions that methanol releases react with the cobalt ions in the solution to form α cobalt hydroxide. Then, α cobalt hydroxide converts to β cobalt hydroxide.
The SEM images of the prepared Co3O4@MSDCN composites are shown in Fig. 2c and d, which confirm that the structure and pore size of the composites were not changed during the facile thermal reactions. A large amount of Co3O4 was directly and uniformly grown on the surface of the MSDCN, which provides highways for electron delivery. In addition, the size of cubic Co3O4 is approximately 1 μm, which supplies abundant space for the redox reactions.11
In Fig. 3a, the X-ray diffraction analysis of the sample can be unambiguously indexed to the cubic spinel Co3O4, which confirms the presence of Co3O4. The diffraction peaks at 19.01°, 31.30°, 36.82°, 38.62°, 44.82°, 59.35° and 65.21° can be indexed as the (111), (220), (311), (222), (400), (511) and (440) crystal planes of Co3O4, respectively. Moreover, there is a broad peak at approximately 2θ = 25° of the XRD spectrum, which is the typical spectrum of amorphous carbon. Thus, the prismatic layer was completely carbonized into an amorphous carbon substrate, which indicates that the cubic Co3O4 was successfully MSDCN substrate without impurity.34 Fig. 3b shows the Raman spectrum of the sample, MSDCN and pure Co3O4 in detail over the range of 0 to 2000 cm−1, where the vibrational peaks at approximately 1347 and 1585 cm−1 can be attributed to the D and G bands.35 The additional peaks below the wavenumber of 700 cm−1 (the peaks at 473, 516 and 676 cm−1) can be assigned to classical vibration modes Eg, F2g, and A1g of spinel oxide Co3O4, respectively.32
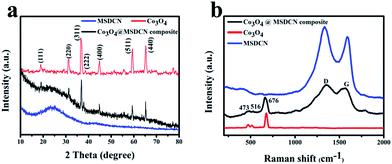 | ||
| Fig. 3 (a) XRD patterns of the Co3O4@MSDCN composites, MSDCN and pure Co3O4. (b) Raman spectra of the Co3O4@MSDCN composites, MSDCN and pure Co3O4. | ||
Transmission electron microscopy (TEM), high-resolution TEM (HRTEM) and selected area electron diffraction (SAED) were further applied to characterize the morphological and structural properties of the Co3O4@MSDCN composites. In Fig. 4a, the TEM image shows a single Co3O4 cube, which is approximately the size of the SEM images. From the HRTEM image in Fig. 4b, the measured lattice space is 0.243 nm, which exactly corresponds to the (311) crystal planes of Co3O4. The inset in Fig. 4b of the selected area electron diffraction (SAED) pattern demonstrates the monocrystalline nature of cubic Co3O4.
By using a set of electrochemical tests, the electrochemical performance of the prepared Co3O4@MSDCN composites is evaluated based on a three-electrode mode with a 6 M KOH solution as the electrolyte. Fig. 5a presents the representative cyclic voltammetry (CV) curves of the Co3O4@MSDCN composites at different potential sweep rates of 5–100 mV s−1 over a potential range of −0.2 to 0.6 V. In terms of shape, the CV curves are consistent with previous studies on Co3O4 in a KOH solution.1,19,36,37 At different scan rates, there is an evident pair of typical redox peaks during the anodic and cathodic sweeps, which confirms the pseudo-capacitive behaviour of Co3O4. The redox peaks consistently occur with the conversion among different cobalt oxidation states. The following reactions describe the process.
| Co3O4 + H2O + OH− ⇔ 3CoOOH + e− | (4) |
| CoOOH + OH− ⇔ CoO2 + H2O + e− | (5) |
The two pairs of peaks that correspond to the above equations merge with each other, so there is only an oxidation peak (A) and a reduction peak (C) in Fig. 5a, which is consistent with a relevant study.32 As the scan rates increase, the CV shapes remain unchanged due to the porous carbon scaffold and macroporous structure, which provide highways for electrons delivery and ion diffusion and result in the superior performance of the electrode.11 Because of the incomplete redox reactions at higher scan rates, the oxidation and reduction peaks shift from 0.10 V and 0.21 V at a scan rate of 5 mV s−1 to 0.04 V and 0.34 V at 100 mV s−1, respectively, in terms of position. At the scan rate of 100 mV s−1, as displayed in Fig. 5c, the CV curve of nickel foam shows that its effect is eliminated. The curve shows that the effect is notably weak and negligible. Additionally, the CV curves of pure Co3O4 and Co3O4@MSDCN composites are presented for comparison in Fig. 5c. It can be concluded that the specific capacitance, which is proportional to the area of the enclosed CV curve, is significantly improved after Co3O4 was compounded with MSDCN because the interconnected structure enhances electron transfer and electrolyte infiltration to result in a higher ion diffusion rate of the electrode.
A series of galvanostatic charge–discharge curves of the Co3O4@MSDCN composites at different current densities are shown in Fig. 5b. According to eqn (1), the capacitance is proportional to the discharge time. The specific capacitance reaches 1307, 1210, 1018, and 875 F g−1 at current densities of 1, 2, 5, and 10 A g−1, respectively. A specific capacitance of 798 F g−1 remains when the current density increases to 20 A g−1. For comparison, Fig. 5d shows the charge–discharge curves of Co3O4@MSDCN composites and pure Co3O4 at a current density of 1 A g−1 (340 F g−1 for pure Co3O4). Evidently, the Co3O4@MSDCN composites have a much higher capacitance than pure Co3O4 because of the application of MSDCN. Fig. 5e presents that the specific capacitance with the increase in current density has a capacity retention of 61.0% at 20 A g−1, which testifies the impressive rate performance.
As a significant property of the electrochemical behaviour of the electrode, the cycling performance of Co3O4@MSDCN composites was tested for 3000 cycles at a current density of 5 A g−1 and shown in Fig. 5f. After 3000 cycles, 84% of the initial capacitance remained, which indicates a superior durability because of the interconnected structure of MSDCN. This interconnected structure simultaneously enhances the capacitance and results in superior durability. The steady durability of the electrode enables application of Co3O4@MSDCN composites in supercapacitor production.
It is acknowledged that the conductivity of the electrode material significantly affects the electrochemical performance of supercapacitors, which was characterized using electrochemical impedance spectroscopy (EIS). In the high frequency region, the intercept at the real axis and the diameter of semicircle signify the resistance of the electrochemical system (Rs) and charge transfer resistance (Rct), respectively. The Nyquist plots in Fig. 6 present that Co3O4@MSDCN composites have much lower Rs and Rct values (1.45 and 7.5 Ω) than pure Co3O4 (1.51 and 30.2 Ω) because MSDCN serves as a highly conductive platform and affords efficient ion transport channels, which proves that the Co3O4@MSDCN composite has a great potential in supercapacitor applications.
 | ||
| Fig. 6 Impedance plot of Co3O4@MSDCN composites, pure Co3O4 and MSDCN, the inset is the magnified impedance plot of Co3O4@MSDCN composites and MSDCN. | ||
The excellent electrochemical performance of Co3O4@MSDCN composites can be attributed to their unique structural feature. First, due to the high conductivity of the inner interconnected structure of MSDCN, it serves as channels for efficient electrons transfer that enables electrons to spread out the entire composite. In addition, the binder-free electrodes provide reliable electrical connections to Co3O4 to accelerate electron transport. Second, the large channel volume of MSDCN ensures efficient proton penetration, while the high surface area of the inner wall is capable of loading and supporting a large amount of cubic Co3O4 for the effective use of the active materials. Therefore, using this unique macroporous carbon network, MSDCN, as a substrate improves the electrical conductivity of Co3O4 and accelerates ion transfer in the electrolyte at the same time. Third, in the process of cycling, MSDCN reinforces the structure to prevent the occurrence of cracks and collapses, which leads to the excellent cycle stability and enhances the cycle life. Last, the uniformly aligned macroporous cubic Co3O4 offers a large surface area, which results in sufficient contact between the active materials and the electrolyte to give the facile electrolyte easy access to a fast redox reaction and significantly enhance the efficiency.
4. Conclusions
As a cathode of supercapacitors, Co3O4@MSDCN composites exhibit an excellent electrochemical performance with high capacitances of 1307 F g−1 and 798 F g−1 at current densities of 1 A g−1 and 20 A g−1, respectively. The capacitance loss is only 16% at 5 A g−1 after 3000 cycles. Such an impressive performance is attributed to the unique and well-organized macroporous structure. Furthermore, MSDCN can be combined with other active materials to enhance the electrochemical performance of supercapacitors and provide significant conductivity and efficient electrolyte penetration. Finally, this approach broadens the scope of research on related materials that can be applied to energy storage devices to widen the scale of utilization.Acknowledgements
This work was financially supported by self-determined research funds of CCNU from the colleges' basic research and operation of MOE (No. CCNU 15A02035 and CCNU 15DZ007), the Key Scientific Project of Wuhan City (No. 2013011801010598), the Scientific Project of AQSIQ (No. 2013IK093) and the National Natural Science Foundation of China (No. 50802032).Notes and references
- J. Lang, X. Yan and Q. Xue, J. Power Sources, 2011, 196, 7841–7846 CrossRef CAS PubMed.
- R. T. Wang, X. B. Yan, J. W. Lang, Z. M. Zheng and P. Zhang, J. Mater. Chem. A, 2014, 2, 12724–12732 CAS.
- X. Xia, D. Chao, Z. Fan, C. Guan, X. Cao, H. Zhang and H. J. Fan, Nano Lett., 2014, 14, 1651–1658 CrossRef CAS PubMed.
- X. Sun, P. Cheng, H. Wang, H. Xu, L. Dang, Z. Liu and Z. Lei, Carbon, 2015, 92, 1–10 CrossRef CAS PubMed.
- B. You, J. Jiang and S. Fan, ACS Appl. Mater. Interfaces, 2014, 6, 15302–15308 CAS.
- L. Li, R. Li, S. Gai, S. Ding, F. He, M. Zhang and P. Yang, Chem.–Eur. J., 2015, 21, 7119–7126 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- X. Cao, Y. Shi, W. Shi, G. Lu, X. Huang, Q. Yan, Q. Zhang and H. Zhang, Small, 2011, 7, 3163–3168 CrossRef CAS PubMed.
- H. B. Li, M. H. Yu, X. H. Lu, P. Liu, Y. Liang, J. Xiao, Y. X. Tong and G. W. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 745–749 CAS.
- H. Jiang, J. Ma and C. Li, Chem. Commun., 2012, 48, 4465–4467 RSC.
- D. Lan, Y. Chen, P. Chen, X. Chen, X. Wu, X. Pu, Y. Zeng and Z. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 11839–11845 CAS.
- C. Zhou, Y. Zhang, Y. Li and J. Liu, Nano Lett., 2013, 13, 2078–2085 CrossRef CAS PubMed.
- P. Yang, Y. Ding, Z. Lin, Z. Chen, Y. Li, P. Qiang, M. Ebrahimi, W. Mai, C. P. Wong and Z. L. Wang, Nano Lett., 2014, 14, 731–736 CrossRef CAS PubMed.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28–E62 CrossRef CAS PubMed.
- X. Wu, Y. Zeng, H. Gao, J. Su, J. Liu and Z. Zhu, J. Mater. Chem. A, 2013, 1, 469–472 CAS.
- X. H. Xia, J. P. Tu, Y. J. Mai, X. L. Wang, C. D. Gu and X. B. Zhao, J. Mater. Chem., 2011, 21, 9319–9325 RSC.
- X. Wang, M. Li, Z. Chang, Y. Yang, Y. Wu and X. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 2280–2285 CAS.
- D. T. Dam and J. M. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 20729–20737 CAS.
- M. J. Deng, F. L. Huang, I. W. Sun, W. T. Tsai and J. K. Chang, Nanotechnology, 2009, 20, 7480 Search PubMed.
- B. R. Duan and Q. Cao, Electrochim. Acta, 2012, 64, 154–161 CrossRef CAS PubMed.
- X. H. Xia, J. P. Tu, Y. P. Zhang, Y. J. Mai, X. L. Wang, C. D. Gu and X. B. Zhao, RSC Adv., 2012, 2, 1835–1841 RSC.
- W. J. Zhou, J. Zhang, T. Xue, D. D. Zhao and H. L. Li, J. Mater. Chem., 2008, 18, 905–910 RSC.
- W. J. Zhou, M. W. Xu, D. D. Zhao, C. L. Xu and H. L. Li, Microporous Mesoporous Mater., 2009, 117, 55–60 CrossRef CAS PubMed.
- W. Xiong, Y. Gao, X. Wu, X. Hu, D. Lan, Y. Chen, X. Pu, Y. Zeng, J. Su and Z. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 19416–19423 CAS.
- Q. Guan, J. Cheng, B. Wang, W. Ni, G. Gu, X. Li, L. Huang, G. Yang and F. Nie, ACS Appl. Mater. Interfaces, 2014, 6, 7626–7632 CAS.
- L. Q. Mai, A. Minhas-Khan, X. Tian, K. M. Hercule, Y. L. Zhao, X. Lin and X. Xu, Nat. Commun., 2013, 4, 2923 Search PubMed.
- M. F. de Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535–539 CrossRef CAS PubMed.
- H. J. Liu, X. M. Wang, W. J. Cui, Y. Q. Dou, D. Y. Zhao and Y. Y. Xia, J. Mater. Chem., 2010, 20, 4223–4230 RSC.
- X. L. Wu, T. Wen, H. L. Guo, S. B. Yang, X. K. Wang and A. W. Xu, ACS Nano, 2013, 7, 3589–3597 CrossRef CAS PubMed.
- S. Yu, D. Liu, S. Zhao, B. Bao, C. Jin, W. Huang, H. Chen and Z. Shen, RSC Adv., 2015, 5, 30943–30949 RSC.
- J. Jang and H. Yang, J. Mater. Sci., 2000, 35, 2297–2303 CrossRef CAS.
- R. B. Rakhi, W. Chen, D. Cha and H. N. Alshareef, Nano Lett., 2012, 12, 2559–2567 CrossRef CAS PubMed.
- H. Zhang, J. Wu, C. Zhai, X. Ma, N. Du, J. Tu and D. Yang, Nanotechnology, 2008, 19, 369–374 Search PubMed.
- J. Xu, L. Gao, J. Cao, W. Wang and Z. Chen, Electrochim. Acta, 2010, 56, 732–736 CrossRef CAS PubMed.
- T. Kuila, S. Bose, A. K. Mishra, P. Khanra, N. H. Kim and J. H. Lee, Prog. Mater. Sci., 2012, 57, 1061–1105 CrossRef CAS PubMed.
- X. Qing, S. Liu, K. Huang, K. Lv, Y. Yang, Z. Lu, D. Fang and X. Liang, Electrochim. Acta, 2011, 56, 4985–4991 CrossRef CAS PubMed.
- J. Liu, J. Jiang, C. Cheng, H. Li, J. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076–2081 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |



