Synthesis, evaluation and thermodynamics of a 1H-benzo-imidazole phenanthroline derivative as a novel inhibitor for mild steel against acidic corrosion†
Abstract
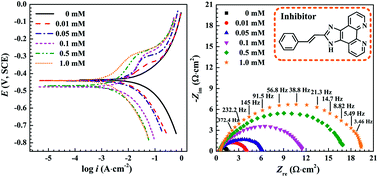
A novel 1H-benzo-imidazole phenanthroline derivative (1) was developed as a corrosion inhibitor for mild steel in 1.0 M HCl solution with temperature ranging from 25 °C to 90 °C. Potentiodynamic polarization, electrochemical impedance spectroscopy and weight loss indicates that inhibitor 1 can efficiently protect mild steel from corrosion in acidic medium with better performance under moderately higher concentration and temperature. Based on thermodynamic and kinetic analysis, it is observed that 1 functioned as a mixed-type inhibitor and obeyed Langmuir adsorption isotherm via chemisorption. Further assisted by synergistic effect of iodide ions, inhibition efficiency can be dramatically enhanced up to 99.2%.

 Please wait while we load your content...
Please wait while we load your content...