A form-stable phase change material made with a cellulose acetate nanofibrous mat from bicomponent electrospinning and incorporated capric–myristic–stearic acid ternary eutectic mixture for thermal energy storage/retrieval
Abstract
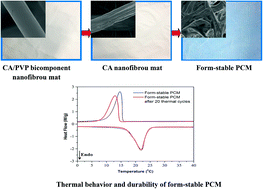
An innovative type of form-stable phase change material (PCM) was prepared by incorporating a capric–myristic–stearic acid (CMS) ternary eutectic mixture with a cellulose acetate (CA) nanofibrous mat that was derived from electrospinning a binary mixture of CA/polyvinylpyrrolidone (PVP) and subsequent selective dissolution of PVP component from the obtained bicomponent nanofibrous mat. PVP removal from the CA/PVP bicomponent nanofibers created nanoporous features on the resultant CA nanofiber surface and increased CMS incorporation capability of the nanofibrous mat. Morphology, thermal behavior and durability, and thermal energy storage/retrieval capacity of the prepared CMS/CA nanofibrous form-stable PCM were investigated. This form-stable PCM could maintain well the PCM characteristics even after multiple thermal cycle uses and demonstrated great thermal storage/retrieval capability and temperature regulation ability.

 Please wait while we load your content...
Please wait while we load your content...