Unravelling the effect of flavonoids on the kinetic profiles of acrylamide in the Maillard reaction
Yu Zhang*ab,
Qiao Wanga,
Mengmeng Huanga and
Xinyu Chena
aDepartment of Food Science and Nutrition, College of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou 310058, Zhejiang, China. E-mail: y_zhang@zju.edu.cn; Fax: +86-571-88982211; Tel: +86-571-88982211
bZhejiang Key Laboratory for Agro-Food Processing, Zhejiang R & D Center for Food Technology and Equipment, Fuli Institute of Food Science, Zhejiang University, Hangzhou 310058, Zhejiang, China
First published on 28th September 2015
Abstract
Acrylamide is mainly generated from asparagine and reducing sugars. The comprehensive kinetic profile of acrylamide and Maillard reaction products (MRP) is important for understanding the control of acrylamide. We unraveled the role of flavonoids in the kinetic process of acrylamide via a potato-based asparagine–glucose microwave heating system. Ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) was employed for the simultaneous determination of asparagine, glucose, fructose and acrylamide. The multi-response non-linear regression models were used for kinetic parameter estimation. The results indicated a positive role of flavonoids in the fructose-participating (k3: 0.461–0.674; P < 0.05, P < 0.01, compared to the control) instead of the glucose-participating Maillard reaction (k1: 0.128–0.148; P > 0.05). The addition of flavonoids significantly reduces the formation of acrylamide during the advanced stage (k4: 2.311–4.327; P < 0.05, P < 0.01) but did not affect its elimination process (k6: 0.076–0.113; P > 0.05). Treatment of flavonoids has no significant impact on the formation of melanoidins (k5: 3.645–4.368; P > 0.05) and retains the original sensory attributes of MRP. These findings favor thinking mitigation strategies via the use of additives during food processing.
Introduction
Acrylamide, a probable carcinogen and neurotoxin found in heat processed food, has raised an international health alarm since 2002.1 Since the public health risk was identified, the chemistry, kinetics and toxicity of acrylamide has received wide attention in recent decades.2–4 As a reactive α,β-unsaturated (conjugated) compound, acrylamide is generated from the Maillard reaction via considering free amino acids (mainly asparagine) and carbonyl compounds (e.g. reducing sugars) as main precursors.5,6 The favourable conditions of acrylamide formation could also induce the generation of Maillard brown products, which have stimulated research interests in the formation profile and mitigation mechanism of acrylamide contaminants in food. Previous reviews comprehensively summarized the characteristic and new research advances in both formation and reduction of acrylamide with the collection of mode-of-action progress.7,8 Although numerous studies investigated influential factors contributing to the formation and reduction of acrylamide, it still lacks of evidence on unravelling how the proposed agents prevent the formation of acrylamide via the asparagine or acrolein pathway.The kinetic analysis as an alternative approach has been used for elucidating the formation and reduction mechanism of acrylamide. In both pure chemical and real food systems, the formation of acrylamide follows several interactive pathways, including the formation of Schiff base, Amadori rearrangement and the formation of 3-aminopropionamide that involve the synthesis and degradation of intermediate compounds. The kinetics of acrylamide formation and elimination has been described by different mathematical models with different degrees of complexity, including the two-step formation/elimination model, the non-isothermal model and the six-step mechanistic model.2,7 The two-step model describing as a first-order formation/elimination kinetic process has been widely applied to monitoring the dynamic process of acrylamide levels in model or food systems.9,10 This model is used for judging whether the formation or elimination stage plays a predominant role in the heat process via calculating their kinetic parameters. Previous studies reported some key influential factors affecting the kinetics of acrylamide, including types of sugars as precursors,11 the competitive reactions with asparagine via other amino acids,12 colour development,13 medium pH,14 and water activity.15 Using flavonoid-rich herb extracts as acrylamide inhibitors, our previous studies demonstrated the use of both antioxidant of bamboo leaves and green tea extracts, which are abundant with flavone C-glycosides and flavonols and derivatives, respectively, could effectively reduce the acrylamide levels in the formation-predominant kinetic stage but not in the elimination-predominant stage.16,17 However, the employment of such kinetic approach is unable to reveal the change of intermediate products by heat processing conditions or mode-of-action sites by the effect of foreign chemical agents or additives. In addition, the non-isothermal kinetic model has rarely been employed because the temperature variation should be further considered.2,7 Only one study reported the elimination of acrylamide using the non-isothermal model.18
To further elucidate the whole kinetic process of acrylamide and its precursors, a mechanism-based model has been employed. This comprehensive model uses 6 kinetic rate constants to profile the dynamic change of substances among precursors, sugar isomers, intermediate compounds, acrylamide and its degradation products and melanoidins (Maillard browning products).19 The model suggests that the impact of temperature on acrylamide formation with fructose is more effective than the impact on acrylamide formation with glucose due to the preceding steps with the formation of the Schiff base,20 which could not be observed when the two-step formation/elimination model is employed. Overall, the 6-step mechanistic model provides further insights of the kinetics of acrylamide in a quantitative way.
The employment of natural antioxidant compounds is highlighted as an effective recipe for the mitigation of acrylamide in food. Flavonoids are a large group of herbal polyphenolics that contain a benzopyrane-based structure with a combined 2- or 3-phenyl group.21 As important phenolic antioxidants, previous studies revealed flavonoid-rich extracts or monomers could effectively reduce acrylamide levels in food or model systems.22–24 However, their roles and mode-of-action in Maillard reaction remain unclear. A further mechanistic study demonstrated naringenin (a characteristic compound of flavanones in the flavonoid family) effectively reduces the formation of acrylamide by directly reacting with its precursors.25 Unfortunately, it is still unknown how flavonoid antioxidants are likely to affect the Maillard reaction in such a 6-step kinetic view. Recently, we have established an ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) method for simultaneously analyzing acrylamide and its precursors and main intermediate products in Maillard reaction products (MRP) without significant interference from matrix effects,26 which provides methodological support for investigating the role of flavonoids in the kinetics of acrylamide. Thus, the aim of this study was to unravel the effect of characteristic flavonoids on the kinetics of acrylamide formation and elimination, and reveal their functional sites in the Maillard reaction.
Experimental
Materials and chemicals
The potato powder (Atlantis variety) was purchased from Sanjiang (Group) Potato Products Co., Ltd (Lintao, China). Genistein, homoorientin (luteolin-6-C-glucoside), luteolin and luteolin-7-O-glucoside were obtained from Extrasynthese (Lyon, France). Quercetin, acrylamide, L-asparagine monohydrate, D-(+)-glucose monohydrate and D-(−)-fructose were purchased from Sigma-Aldrich (St. Louis, MO, USA). The chemical structures of flavonoids selected in this study are shown in Fig. 1. D3-acrylamide, 15N2-asparagine and 13C6-glucose (all isotope purity ≥99%) were provided by Cambridge Isotope Laboratories (Andover, MA, USA). Methanol and formic acid were of HPLC grade while other chemicals used in this study were of analytical grade. | ||
| Fig. 1 Chemical structures of flavonoids selected in this study. (A) Homoorientin (luteolin-6-C-glucoside); (B) luteolin; (C) luteolin-7-O-glucoside; (D) genistein; (E) quercetin. | ||
Determination of asparagine and glucose in potato powder
The potato powder (1 g) was sampled and extracted with a phosphate buffer (0.1 mol l−1, pH 6.80). Then, the mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. The supernatant was collected and subjected to the instrumental analysis. To initially measure Maillard reaction precursors, asparagine and glucose contents in potato powder were determined by HPLC, which was performed on a Waters 2695 HPLC chromatograph (Milford, MA, USA). The chromatographic separation was conducted with a Capcell Pak C18 A.Q column (5 μm, 150 mm × 2.0 mm i.d.) (Shiseido, Japan). Samples (30 μl) were eluted with aqueous acetonitrile (75%, v/v) at a flow speed of 1.0 ml min−1. Asparagine was monitored at 254 nm with a diode array detector while glucose was detected with a differential refractive index detector.
000 rpm for 5 min. The supernatant was collected and subjected to the instrumental analysis. To initially measure Maillard reaction precursors, asparagine and glucose contents in potato powder were determined by HPLC, which was performed on a Waters 2695 HPLC chromatograph (Milford, MA, USA). The chromatographic separation was conducted with a Capcell Pak C18 A.Q column (5 μm, 150 mm × 2.0 mm i.d.) (Shiseido, Japan). Samples (30 μl) were eluted with aqueous acetonitrile (75%, v/v) at a flow speed of 1.0 ml min−1. Asparagine was monitored at 254 nm with a diode array detector while glucose was detected with a differential refractive index detector.
Preparation of potato-based asparagine–glucose Maillard reaction system
The stock solutions of asparagine (0.2 mol l−1) and glucose (0.5 mol l−1) were prepared in a phosphate buffer (0.1 mol l−1, pH 6.80). The working concentrations of both precursor substances were set to a final equimolar level (0.14 mol l−1) via diluting their stock solutions with the phosphate buffer as well as taking original contents of asparagine and glucose in the potato powder into consideration. An aliquot of potato powder (10 g) can be configured into a 200 ml of Maillard reaction solution.Mimicking the process of Maillard reaction
Current equimolar asparagine–glucose Maillard reaction was mimicked via a laboratory-level microwave heating system using the Ethos D microwave digestion labstation (MDL) (Milestone Inc., Shelton, CT, USA). Details regarding the operation protocol and working mechanism of MDL were reported in our previous work.23 In this labstation, there is a group of microwave sample vessels in the carousel, which simultaneously allows 1 control group and 5 flavonoid-treatment groups for the Maillard reaction under identical thermal conditions. A routing gradient temperature programming of the MDL is conducted before starting current microwave heating, which is shown as follows: room temperature → 120 °C (200 W, 5 min); 120 °C → 180 °C (500 W, 5 min). The fluctuation range of heating temperature is within ±1 °C. In our preliminary test, the maximum pressure of the asparagine–glucose reaction was determined as 950 kPa during the process of MDL running, which was safe (maximal safety pressure: 3500 kPa) to conduct current experiments.The effect of selected flavonoids on kinetics of acrylamide
The kinetics of acrylamide and its precursors and intermediates via the effect of 5 flavonoids were investigated with the above mimic system. The addition level (0.1 μmol l−1) of flavonoids was selected, which shows maximal reduction effect on acrylamide formation according to our previous dose–response studies.23,27 An aliquot (100 μl) of individual flavonoid (0.1 μmol l−1) was added into a potato-based equimolar asparagine–glucose solution (10 ml final) in each experimental group, while the same volume of phosphate buffer was employed in the control group. The mixed reaction solutions in sealed MDL vessels in both control and experimental groups were simultaneously treated at 180 °C via the MDL with different duration time (0.1, 1, 2, 3, 4, 5, 7.5, 10, 15, 25 and 40 min, 11 treatments in total) after conducting a routine MDL temperature programming. The Maillard reaction of the asparagine–glucose–flavonoid mixture in each test was repeated in triplicates (n = 3). At the end of heat processing, the vessels containing final MRP were taken out from MDL and immediately cooled in prepared ice water to prevent further reactions. The entire cooling procedure was conducted in a studio with stable room temperature (20 °C) as well as air-conditioning.Simultaneous determination of asparagine, glucose, fructose and acrylamide
A mixed internal standard (IS) solution of 15N2-asparagine (100 μg ml−1), 13C6-glucose (1000 μg ml−1) and D3-acrylamide (10 μg ml−1) was prepared in advance. An aliquot (200 μl) of MRP was sampled from the vessels and diluted to 10 ml with the phosphate buffer. An aliquot (2 ml) of the diluent was spiked with the mixed IS solution (500 μl). Then, each vortex-mixed sample solution was directly filtered through 0.22 μm membrane or treated by a solid-phase extraction (SPE) procedure,26 depending on the complexity of sample matrices. The treated samples were finally ready for UHPLC-MS/MS analysis according to our previously reported method.26 The chromatographic separation was performed using a Hypercarb column (100 mm × 2.1 mm i.d., 5 μm, Thermo Electron, San Jose, CA, USA). The MS/MS detection was conducted on a Quattro Ultima triple-quadrupole mass spectrometer (Micromass Company Inc., Manchester, UK) using the electrospray ionization (ESI) source. Asparagine and 15N2-asparagine were detected under the negative ion mode due to matrix interferences under the positive ion mode. All other analytes including their isotope compounds were monitored under the positive ion mode.Determination of melanoidins
The melanoidins in final products were spectrophotometrically measured at a maximal visible absorption (470 nm).19 The concentration of melanoidins was calculated from the Lambert–Beer equation, employing a coefficient of 282 l mol−1 cm−1 and a 1 cm cuvette path length.28Kinetic modelling and statistical analysis
The kinetic modelling and data analysis followed the profile of 6-step mechanistic model. The overall kinetics of acrylamide formation and elimination can be formulated by a schedule of 6 consecutive reactions in which k1–k6 represent the rate constants of the asparagine–glucose reaction, isomer conversion from glucose into fructose, asparagine–fructose reaction, acrylamide formation, melanoidins formation and acrylamide degradation, respectively (Fig. 2). The intermediate process of Maillard reaction is modulated as a representative of Schiff base.19 Corresponding kinetic equations are shown as follows:
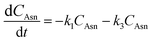 | (1) |
 | (2) |
 | (3) |
 | (4) |
 | (5) |
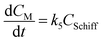 | (6) |
 | (7) |
 | ||
| Fig. 2 Kinetic model of acrylamide formation and elimination used in this study for mimicking the potato-based asparagine–glucose Maillard reaction. k1–k6, kinetic parameters. | ||
Among these kinetic equations, CAsn, CGlu, CFru, CSchiff, CAA, CM and CD indicate the concentrations of asparagine, glucose, fructose, Schiff base, acrylamide, melanoidins and degradation products, respectively, while t represents the reaction time. The initial conditions were set as follows: t = 0, CAsn = 0.14 mol l−1, CGlu = 0.14 mol l−1, CFru = 0, CSchiff = 0, CAA = 0, CM = 0 and CD = 0.
The concentrations of all analytes were expressed as mean ± standard deviation (SD). The kinetic parameters (k1–k6) were estimated by the Marquardt nonlinear least squares regression method and calculated by the SAS v8.2 program (SAS Institute Inc., Beijing, China). The significance was evaluated using the Student's t-test via considering values in the control group as reference levels.
Results and discussion
Specificity of current Maillard reaction system
Current predesigned reaction system employs an equimolar asparagine–glucose binary model system for mimicking the Maillard reaction in an effective way. The reaction is performed under a microwave heating condition via MDL for mimicking favourable generation of acrylamide in conditions of high reaction temperature and short reaction time. Besides, the potato matrix in the reaction system is used, which is in favour of acrylamide formation and close to the real food system. Overall, the equimolar binary model system, microwave heating and the employment of potato matrix result in the specificity of current Maillard reaction system used for the present kinetic study.Simultaneous determination of asparagine, glucose, fructose and acrylamide
A typical chromatogram of asparagine, glucose, fructose and acrylamide and their IS levels in samples is shown in Fig. 3. Although the sample matrix induces an ionization suppression of both positive and negative precursor ions as described previously,26 the analyte peaks in all investigated ion channels do not interfere with foreign impurities. The quantitative results have been corrected through the use of isotopically labelled IS. Thus, the chromatographic quantification of asparagine, glucose, fructose and acrylamide was analysed within 5.5 min per sample. Early study established a mechanistic model for elucidating the kinetics of acrylamide via using LC-MS/MS, high-performance liquid chromatography (HPLC) with refractive index detector and amino acid analysis kit to analyse the contents of acrylamide, reducing sugars and asparagine, respectively.19 Recent kinetic studies also employed LC-MS/MS and/or HPLC with different detectors for analysing these compounds separately.29,30 However, such analytical task takes considerable time and labour and also requires large sample amounts. Furthermore, the limit of quantification (LOQ) of the HPLC method is too restrictive to be competent for trace analysis such as refractive index detection for reducing sugars. Compared with these studies, our UHPLC-MS/MS method provides an effective way to the simultaneous investigation of acrylamide and its precursors.Kinetics of asparagine and glucose affected by flavonoids
The kinetic profiles of asparagine and glucose in both control and experimental groups are shown in Fig. 4A and B. As expected, the concentrations of both reactants decline with the increase of heating duration time, while their degradation rates also decrease over time. In detail, both reactants in all groups undergo steep decline during initial 10 min microwave heating. For instance, the concentrations of asparagine and glucose in control groups reduce by 90.5% and 95.7%, respectively, compared with their initial concentrations prior to Maillard reaction. The loss of asparagine is slower compared with the loss of glucose, which may possibly be ascribed to the re-formation of asparagine from condensation products such as the Amadori rearrangement product and the possible formation of diglucosylamine.19,29 Interestingly, the uses of all selected flavonoids in experimental groups slower the above steep decline of both asparagine and glucose. Compared with initial addition levels of both reactants, their concentrations in experimental groups after 10 min heating reduce by the range of 71.1–81.0% and 87.7–90.7%, respectively, depending on types of flavonoids used. It is well-documented that flavonoids represent the most widely distributed group of plant polyphenols. The selected flavonoids in this study derive from main subclasses including flavone aglycones, flavone glycosides, flavonols and isoflavones, which are capable of trapping reactive carbonyls or dicarbonyl groups and thus competitively inhibiting the Maillard reaction between asparagine and glucose.31,32 The possible mechanism has been found that C-6 and/or C-8 of A-ring in the chemical structures of flavonoids (Fig. 1) may act as functional sites and bond with reactive carbonyl or aldehyde groups, the depletion of which reduces the conversion from decarboxylated Schiff base into 3-aminopropionamide (3-APA, the direct precursor of acrylamide).25,33 At the end stage of kinetics, the concentrations of both asparagine and glucose in the control group nearly come down to zero after 40 min thermal reaction as expected. Nevertheless, asparagine levels in flavonoid treatment groups remain 5.4–11.3% of their original participating levels, while glucose levels remain 2.7–7.2%, depending on types of flavonoids used.Kinetics of fructose affected by flavonoids
The effect of flavonoids on the kinetic behaviour of fructose is shown in Fig. 4C. The concentrations of fructose in all groups increase steeply and achieve maximal levels during the initial 1 min microwave heating. Then, its kinetic profile follows a similar decline way like asparagine and glucose. Overall, flavonoids play an important role in both steep increase and exponential decline stages of the kinetic process of fructose. All 5 flavonoids not only effectively reduce rapid generation of fructose deriving from glucose, but also prevent the participation of fructose in Maillard reaction. Compared with the generation of fructose in the control group, the concentrations of fructose in flavonoid treatment groups reduce by the range of 4.7–16.7% during the initial 1 min heating. Similarly, current kinetic reaction nearly depletes all fructose deriving from glucose isomerization in the control group at the end of 40 min heating. However, fructose levels in various flavonoid treatment groups remain 6.0–16.6% of its maximal generation levels, indicating the reduction effect of flavonoids on the fructose-participating Maillard reaction. Current selected flavonoids exert better reduction effect on the formation of fructose-deriving MRP than the formation of glucose-deriving products in comparison. These results may be ascribed to different kinetic behaviours between ketose and aldose sugars, both of which significantly contribute to the formation of acrylamide.34 Based on up-to-date knowledge of formation mechanism of acrylamide, aldose sugars like glucose are converted to a transient intermediate in a two-stage process which ultimately leads to acrylamide formation, whereas ketose sugars such as fructose are converted to the same intermediate in a one-stage process.30,35Kinetics of acrylamide affected by flavonoids
The kinetics of acrylamide generation and elimination in different groups are shown in Fig. 4D. Acrylamide in all groups undergoes a generation-predominant stage during the initial 4 min heating followed by an elimination-predominant stage afterwards. Compared with the generation of acrylamide in control group, the concentrations of acrylamide in flavonoid treatment groups reduce by the range of 51.0–59.9% during the initial 4 min heating. All investigated flavonoids may reduce the concentrations of acrylamide during the generation-predominant kinetic stage but not affect the concentrations during the elimination-predominant kinetic stage, which are in agreement with our previous work regarding flavonoid-rich extracts.16,17 Recently, many studies reported the reduction effect of polyphenols and phenolic antioxidant extracts on the formation of acrylamide in model or food systems.36–38 However, few studies focus on the role of plant polyphenols and their functional sites in the formation and/or elimination of acrylamide on a kinetic basis. The phenolic structures of flavonoids are known to interfere with the Maillard reaction through scavenging of reactive dicarbonyl compounds such as decarboxylated Amadori product, which is an important intermediate present in the conversion from Schiff base into acrylamide.39,40 In the present work, results of current kinetic investigations regarding the alternation of kinetic stages via the effect of flavonoids are in accordance with the above mechanism.Kinetics of melanoidins affected by flavonoids
The kinetic change of melanoidins in the presence of flavonoids was also investigated (Fig. 4E). The generation of melanoidins in all groups follows a logarithmic profile, which exhibits a steep growth during the initial 4 min heating followed by a gentle increase during the remaining heating time. Melanoidins as major MRP are generated through aldol condensations and polymerization of carbonyl compounds and considered as a main indicator of the browning state.41 The formation of melanoidins and brown colour development are associated with acrylamide contents in MRP, which have been used for the estimation of acrylamide in foods.42,43 Compared with the formation of melanoidins in the control group, the concentrations of melanoidins in flavonoid treatment groups reduce by the range of 0.3–17.8% during the initial 4 min steep increase stage. However, neither of flavonoids could significantly alter final formation of melanoidins at the end of current Maillard reaction with 40 min heating duration (all P > 0.05). In a kinetic view, all investigated flavonoids may reduce the colour development during the steep growth stage but not affect the browning process during the gentle increase stage.Kinetic modelling and related parameters
The kinetic data were fit and related kinetic parameters (k1–k6) were calculated by the Marquardt nonlinear least squares regression. The comparison of kinetic parameter estimation between the control group and either of the flavonoid treatment groups is shown in Table 1. Both generation and elimination processes of fructose (k2 and k3) in all flavonoid treatment groups are significantly reduced if compared with the kinetics in the control group (P < 0.05, P < 0.01), indicating a substantial role of selected flavonoids in the fructose-participating Maillard reaction. It is well-documented that fructose has a higher reactivity with asparagine in the generation of acrylamide than glucose by such a multi-response modelling.20 Recent study also reported the reaction route from glucose is bimolecular, involving the participation of an amino acid like asparagine, whereas fructose is also capable of undergoing a unimolecular reaction to the intermediate Maillard reaction products without the involvement of amino acids.30 Our findings show higher rate constants (k2 and k3) of fructose-participating reactions than related parameter (k1) of glucose-participating reactions, which are in accordance with the above elucidation. Furthermore, it is interesting that flavonoids prefer affecting both formation of fructose from glucose isomerization and elimination of fructose for generating Schiff base intermediate products, indicating flavonoids as reactive phenolic antioxidants play a role in high reactive substances in the occurrence of Maillard reaction. Thus, the role of flavonoids does not seem significant in the asparagine–glucose reaction for generating Schiff base (k1, all P > 0.05).| Group | Kinetic parametersa | |||||
|---|---|---|---|---|---|---|
| k1 (min−1) | k2 (min−1) | k3 (min−1) | k4 (min−1) | k5 (min−1) | k6 (min−1) | |
| a Data were expressed as mean ± SD in triplicates (n = 3) and fit by the Marquardt nonlinear least squares regression method. *P < 0.05, **P < 0.01.b Luteolin-7OG, luteolin-7-O-glucoside. | ||||||
| Control | 0.156 ± 0.032 | 0.673 ± 0.011 | 0.733 ± 0.009 | 5.101 ± 0.421 | 4.487 ± 0.365 | 0.096 ± 0.009 |
| Genistein | 0.146 ± 0.019 | 0.570 ± 0.010** | 0.674 ± 0.052* | 4.327 ± 0.377* | 4.352 ± 0.332 | 0.113 ± 0.030 |
| Homoorientin | 0.133 ± 0.035 | 0.514 ± 0.035** | 0.518 ± 0.061** | 2.817 ± 0.564** | 3.967 ± 0.971 | 0.080 ± 0.017 |
| Luteolin | 0.145 ± 0.063 | 0.523 ± 0.056* | 0.535 ± 0.067* | 3.247 ± 0.489** | 4.135 ± 0.488 | 0.110 ± 0.027 |
| Luteolin-7OGb | 0.148 ± 0.032 | 0.555 ± 0.052* | 0.578 ± 0.091* | 3.515 ± 0.687* | 4.368 ± 0.560 | 0.089 ± 0.054 |
| Quercetin | 0.128 ± 0.044 | 0.497 ± 0.018** | 0.461 ± 0.026** | 2.311 ± 0.087** | 3.645 ± 0.978 | 0.076 ± 0.010 |
Besides the role in the kinetics of fructose, the addition of flavonoids is able to significantly reduce the formation of acrylamide during the advanced reaction stage (k4, P < 0.05), but is not able to significantly affect the elimination of acrylamide (k6, P > 0.05). Recently, some colleagues investigated the impact of reaction condition changes on the generation of acrylamide in a kinetic view. An early study reported a decline of pH in both model system and potato-based matrix is associated with both formation and elimination kinetics of acrylamide using the first-order kinetic model.14 Bassama et al.29 revealed an increase in reaction rates of acrylamide formation and elimination with decreasing water activity using a single response modelling. Isleroglu et al.44 found steam-assisted baking at 165 °C reduces the first-order reaction kinetics of acrylamide formation and browning kinetics in cookies, while keeping the sensory quality. However, the study based on a first-order modelling approach could not further insight the kinetic behaviour of acrylamide formation in detailed steps of Maillard reaction affected by various changeable factors such as pH, heating style and the use of additives. Thus, current work provides further evidence on the reduction of acrylamide and advanced recognition for the role of additives such as flavonoids.
The treatment of flavonoids has no significant impact on the formation of melanoidins (k5, all P > 0.05). As final MRP, melanoidins could either impair or improve the overall quality of processing foods.45 These findings indicated the treatment of flavonoids could not only effectively reduce the concentrations of acrylamide during its generation-predominant stage, but also keep the acceptance of original sensory attributes. Our previous study used the artificial neural network (ANN) model to predict the reduction of acrylamide with the effect of flavonoids via taking changes of antioxidant capacity of MRP as variables, indicating a good connection of acrylamide reduction with antioxidant property of MRP.27 Besides, some other studies also reported a positive correlation between antioxidant capacity and acrylamide contents in MRP.32,46 In a mechanistic view, flavonoids as phenolic antioxidants may be oxidized at high temperature to generate quinones and free radicals that eliminate the formation of acrylamide in final MRP.47 Nevertheless, antioxidants generated from Maillard reaction such as melanoidins may inhibit the formation of free radicals and thus prevent their attack to acrylamide. In a view of kinetic behaviour in the present study, flavonoids did not seem ultimately affect the formation of melanoidins though their reduction effect on melanoidins were observed in the kinetic progress (Fig. 4E).
Current kinetic study affected by flavonoids was investigated by a 4-step experiment procedure, including the mimicking of Maillard reaction, sample treatment, UHPLC-MS/MS analysis and spectrophotometric measurement of melanoidins. The operation of MDL for the generation of each MRP costs 10 min for a routing gradient temperature programming and 0.1–40 min for kinetic reaction, depending on the duration time of Maillard reaction. The rapid sample treatment of MRP includes IS spiking and appropriate dilution without liquid–liquid extraction or further clean-up (∼10 min). The UHPLC-MS/MS analysis costs 5.5 min per run. The measurement of melanoidins includes an appropriate sample dilution and spectrophotometric analysis can also be regarded as a rapid process (∼10 min). Taken together, the overall experimental duration for investigating the kinetics of Maillard reaction ranges approximately 40–80 min.
Conclusions
In summary, we established a multi-response model and investigated the effect of flavonoids on the kinetic behaviour of acrylamide and its precursors and key intermediates during the asparagine–glucose Maillard reaction via microwave heating approach. Our findings indicated a positive role of flavonoids in the fructose-participating Maillard reaction rather than the glucose-participating reaction, including the conversion from glucose into fructose and the formation of intermediate products from asparagine–fructose interaction. The addition of flavonoids could significantly reduce the formation of acrylamide during the advanced reaction stage but not significantly affect its elimination process. As a result, the treatment of flavonoids has no significant impact on the formation of melanoidins, indicating the original sensory attributes of MRP especially the colour development should not be changed. Taken together, we estimated kinetic parameters to unravel an understanding on how flavonoids affect the formation and elimination of acrylamide and MRP, which is favour of thinking mitigation strategies during food processing. Future work will consider using the conventional fluorescence imaging probes to investigate the kinetics of acrylamide formation and elimination in the complex Maillard reaction.Acknowledgements
We gratefully acknowledged financial supports from the Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholars of China (Grant No. LR12C20001) and the National Natural Science Foundation of China (Grant No. 21277123).References
- E. Tareke, P. Rydberg, P. Karlsson, S. Eriksson and M. Törnqvist, J. Agric. Food Chem., 2002, 50, 4998–5006 CrossRef CAS PubMed.
- M. G. Corradini and M. Peleg, Crit. Rev. Food Sci. Nutr., 2006, 46, 489–517 CrossRef CAS PubMed.
- D. R. Lineback, J. R. Coughlin and R. H. Stadler, Annu. Rev. Food Sci. Technol., 2012, 3, 15–35 CrossRef CAS PubMed.
- M. Friedman, Food Funct., 2015, 6, 1752–1772 CAS.
- D. S. Mottram, B. L. Wedzicha and A. T. Dodson, Nature, 2002, 419, 448–449 CrossRef CAS PubMed.
- R. H. Stadler, I. Blank, N. Varga, F. Robert, J. Hau, P. A. Guy, M.-C. Robert and S. Riediker, Nature, 2002, 419, 449–450 CrossRef CAS PubMed.
- Y. Zhang, Y. Ren and Y. Zhang, Chem. Rev., 2009, 109, 4375–4397 CrossRef CAS PubMed.
- Y. Zhang and Y. Zhang, Crit. Rev. Food Sci. Nutr., 2007, 47, 521–542 CrossRef CAS PubMed.
- K. Franke, U. Strijowski and E. H. Reimerdes, J. Food Eng., 2009, 90, 135–140 CrossRef CAS PubMed.
- J. J. Knol, G. Å. I. Viklund, J. P. H. Linssen, I. M. Sjöholm, K. I. Skog and M. A. J. S. van Boekel, Food Chem., 2009, 113, 103–109 CrossRef CAS PubMed.
- K. de Vleeschouwer, I. van der Plancken, A. van Loey and M. E. Hendrickx, Food Chem., 2009, 114, 116–126 CrossRef CAS PubMed.
- K. de Vleeschouwer, I. van der Plancken, A. van Loey and M. E. Hendrickx, Food Chem., 2009, 114, 535–546 CrossRef CAS PubMed.
- F. Pedreschi, O. Bustos, D. Mery, P. Moyano, K. Kaack and K. Granby, J. Food Eng., 2007, 79, 989–997 CrossRef PubMed.
- K. de Vleeschouwer, I. van der Plancken, A. van Loey and M. E. Hendrickx, J. Agric. Food Chem., 2006, 54, 7847–7855 CrossRef CAS PubMed.
- K. de Vleeschouwer, I. van der Plancken, A. van Loey and M. E. Hendrickx, Biotechnol. Prog., 2007, 23, 722–728 CrossRef CAS PubMed.
- Y. Zhang, T. Ying and Y. Zhang, J. Food Sci., 2008, 73, C60–C66 CrossRef CAS PubMed.
- Y. Zhang and Y. Zhang, J. Food Eng., 2008, 85, 105–115 CrossRef CAS PubMed.
- E. Kolek, P. Šimon and P. Šimko, J. Food Sci., 2007, 72, E341–E344 CrossRef CAS PubMed.
- J. J. Knol, W. A. M. van Loon, J. P. H. Linssen, A.-L. Ruck, M. A. J. S. van Boekel and A. G. J. Voragen, J. Agric. Food Chem., 2005, 53, 6133–6139 CrossRef CAS PubMed.
- J. J. Knol, J. P. H. Linssen and M. A. J. S. van Boekel, Food Chem., 2010, 120, 1047–1057 CrossRef CAS PubMed.
- Z. Chen, S. Zheng, L. Li and H. Jiang, Curr. Drug Metab., 2014, 15, 48–61 CrossRef CAS.
- Y. Zhang, J. Chen, X. Zhang, X. Wu and Y. Zhang, J. Agric. Food Chem., 2007, 55, 523–528 CrossRef CAS PubMed.
- Y. Zhang, X. Chen, J. Cheng, C. Jin and Y. Zhang, RSC Adv., 2014, 4, 24147–24155 RSC.
- F. Zhu, Y.-Z. Cai, J. Ke and H. Corke, J. Sci. Food Agric., 2009, 89, 1674–1681 CrossRef CAS PubMed.
- K.-W. Cheng, X. Zeng, Y. S. Tang, J.-J. Wu, Z. Liu, K.-H. Sze, I. K. Chu, F. Chen and M. Wang, Chem. Res. Toxicol., 2009, 22, 1483–1489 CrossRef CAS PubMed.
- Y. Zhang, Y. Ren, J. Jiao, D. Li and Y. Zhang, Anal. Chem., 2011, 83, 3297–3304 CrossRef CAS PubMed.
- J. Cheng, X. Chen, S. Zhao and Y. Zhang, Food Chem., 2015, 168, 90–99 CrossRef CAS PubMed.
- S. I. F. S. Martins and M. A. J. S. van Boekel, Food Chem., 2005, 90, 257–269 CrossRef CAS PubMed.
- J. Bassama, P. Brat, P. Bohuon, B. Hocine, R. Boulanger and Z. Günata, Food Res. Int., 2011, 44, 1452–1458 CrossRef CAS PubMed.
- J. K. Parker, D. P. Balagiannis, J. Higley, G. Smith, B. L. Wedzicha and D. S. Mottram, J. Agric. Food Chem., 2012, 60, 9321–9331 CrossRef CAS PubMed.
- C. Jin, X. Wu and Y. Zhang, Food Res. Int., 2013, 51, 611–620 CrossRef CAS PubMed.
- Y. Liu, P. Wang, F. Chen, Y. Yuan, Y. Zhu, H. Yan and X. Hu, Food Chem., 2015, 186, 46–53 CrossRef CAS PubMed.
- V. M. Totlani and D. G. Peterson, J. Agric. Food Chem., 2006, 54, 7311–7318 CrossRef CAS PubMed.
- V. Gökmen and H. Z. Şenyuva, Food Addit. Contam., 2006, 23, 348–354 CrossRef PubMed.
- B. L. Wedzicha, D. S. Mottram, J. S. Elmore, G. Koutsidis and A. T. Dodson, Adv. Exp. Med. Biol., 2005, 561, 235–253 CrossRef CAS.
- K. Kotsiou, M. Tasioula-Margari, E. Capuano and V. Fogliano, Food Chem., 2011, 124, 242–247 CrossRef CAS PubMed.
- D. Li, Y. Chen, Y. Zhang, B. Lu, C. Jin, X. Wu and Y. Zhang, J. Food Sci., 2012, 77, C1144–C1149 CrossRef CAS PubMed.
- R. A. Oral, M. Dogan and K. Sarioglu, Food Chem., 2014, 142, 423–429 CrossRef CAS PubMed.
- P. V. Guerra and V. A. Yaylayan, J. Agric. Food Chem., 2014, 62, 3831–3836 CrossRef CAS PubMed.
- R. H. Stadler, F. Robert, S. Riediker, N. Varga, T. Davidek, S. Devaud, T. Goldmann, J. Hau and I. Blank, J. Agric. Food Chem., 2004, 52, 5550–5558 CrossRef CAS PubMed.
- J. Pagán, A. Ibarz, L. Elvira, J. Trillo, S. de Frutos and A.-P. Echavarría, Food Res. Int., 2012, 48, 802–807 CrossRef PubMed.
- V. Gökmen, Ö. Ç. Açar, G. Arribas-Lorenzo and F. J. Morales, J. Food Eng., 2008, 87, 380–385 CrossRef PubMed.
- H. Lu and H. Zheng, Food Chem., 2012, 134, 2521–2525 CrossRef CAS PubMed.
- H. Isleroglu, T. Kemerli, M. Sakin-Yilmazer, G. Guven, O. Ozdestan, A. Uren and F. Kaymak-Ertekin, J. Food Sci., 2012, 77, E257–E263 CrossRef CAS PubMed.
- R. Zamora and F. Hidalgo, Crit. Rev. Food Sci. Nutr., 2007, 45, 49–59 CrossRef PubMed.
- S. Ou, J. Shi, C. Huang, G. Zhang, J. Teng, Y. Jiang and B. Yang, J. Hazard. Mater., 2010, 182, 863–868 CrossRef CAS PubMed.
- R. M. Vinci, F. Mestdagh and B. D. Meulenaer, Food Chem., 2012, 133, 1138–1154 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |


