An immobilized molybdenum acetylacetonate complex on expanded starch for the epoxidation of stillingia oil†
Abstract
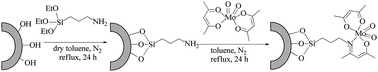
An active heterogeneous catalyst, namely a molybdenum acetylacetonate complex immobilized on expanded corn starch (ECS-MoO2(acac)2), was prepared and its catalytic activity for epoxidation of stillingia oil with tert-butyl hydroperoxide (TBHP) was investigated. The heterogeneous catalysts were characterized using inductively coupled plasma optical emission spectrometry, Fourier transform infrared spectroscopy, thermogravimetry and differential thermal analyses, scanning electron microscopy, N2 adsorption–desorption and X-ray photoelectron spectroscopy. By using this catalyst, an environmentally benign process for epoxide production in a heterogeneous manner was developed. The catalyst could be recovered easily and reused without significant degradation in its activity for at least 5 times.

 Please wait while we load your content...
Please wait while we load your content...