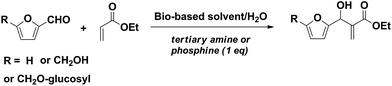
Bio-based solvents for the Baylis–Hillman reaction of HMF†
Jia-Neng Tanab,
Mohammed Ahmara and
Yves Queneau*a
aUniversité de Lyon, Institut de Chimie et Biochimie Moléculaires et Supramoléculaires, ICBMS, UMR5246, CNRS, Université Lyon 1, INSA-Lyon, CPE Lyon, INSA Lyon, Bât. Jules Verne, 20 Avenue A. Einstein, Villeurbanne F-69621, France. E-mail: yves.queneau@insa-lyon.fr
bTobacco Research Institute of The Chinese Academy of Agricultural Sciences, Qingdao 266101, P. R. China
First published on 5th August 2015
Abstract
The Baylis–Hillman reaction of HMF was investigated in various bio-based solvent systems and in water. Although pure water is able to promote the reaction, aqueous mixtures with ethanol, isopropanol, methyl-THF (MeTHF), tetrahydrofurfuryl-alcohol (THFA) show better overall efficacy for the HMF Baylis–Hillman reaction than pure water or the pure bio-based solvent. Such solvent systems can replace THF or dioxane, often used for BH reactions. However, pure bio-based solvents can be used in the case of the more polar glucosyloxymethylfurfural (GMF). These results show that the most appropriate medium must also take into account the polarity of the starting aldehyde.
Introduction
Bio-based chemistry has become an important topic for academic and industrial chemical research, and in this field, carbohydrates, being abundant and cheap carbon renewable resources, play a major role.1 A list of platform molecules with a wide range of potential applications has been established by Bozell and Petersen, including 5-hydroxymethyl furfural (HMF), furfural, γ-valerolactone, glycerol, succinic acid, 2-methyl-THF (MeTHF) and lactic acid.2 Among them, HMF (and its ensuing substituted furan derivatives) appears as one of the most attractive bio-based chemicals and a key building block for transforming biomass-derived oxygenated hydrocarbons into fuels and chemicals,2,3 notably through numerous possible transformations of its aldehyde function. Curiously, very little attention has been paid to the Baylis–Hillman reaction of HMF, despite all the advantages that this reaction theoretically shows: (i) the use of commercially available starting materials; (ii) its favorable atom economy; (iii) densely functionalized products with the possible creation of a new chiral center; (iv) a wide range of promoters and catalysts, possibly avoiding the utilization of any heavy metals; (v) mild reaction conditions. Referred to as Morita–Baylis–Hillman, this reaction involves the α-position of an activated double bond and a sp2 electrophilic carbon (most often an aldehyde) and it is promoted by a tertiary amine or an alkylphosphine.4Concerning the solvent, it has been reported that the MBH reaction could proceed in the presence of water, inducing either beneficial hydrophobic effect or stabilization of the zwitterionic intermediate.5 Water can also act as a proton donor during the proton-transfer step of the reaction, which has been proposed as the rate-determining step based on kinetic and theoretical studies for the reaction in aqueous or other protic media.6 Hence, aqueous media (especially homogeneous H2O/solvent medium) have often been used for this reaction to improve the result in terms of yields and reaction time. However, apart from simple alcohols, no bio-based solvents (considered as emerging media for the design of eco-efficient processes)7 have been included in previous studies, either for HMF or for any other MBH substrate. Though solvent-free chemical processes would be ideal, in many cases solvents are necessary for improving mass and heat transfer and they may contribute to the reaction though their specific effects. Investigating new media with a lower environmental impact is thus useful. Compared to common organic solvents, commonly used for chemical transformations, bio-based solvents, showing low toxicity, low vapour pressure and good biodegradability, are regarded as alternative “green solvents” in organic synthesis.7b,f,g In keeping with our program on biobased MBH products for which we reported a first account on the use of glucosyloxymethylfurfural (GMF),8 we then investigated, from a synthetic point of view, the MBH reaction of HMF in various bio-based solvents and their mixtures with water. For the purpose of comparing the influence of the substrate polarity, two analogues, the less polar furfural and the more polar GMF were also used in the study.
Results and discussion
As a model reaction, the 1,4-diazabicyclo[2.2.2]octane (DABCO) promoted Baylis–Hillman reaction of 5-hydroxymethyl furfural (HMF, 1) with ethyl acrylate (2), in different bio-based media, was studied. First a rapid screening of several systems was performed by estimating the efficiency of the reaction, as measured by the adduct/HMF ratio followed by NMR. Though this measurement is not able to provide a complete picture of the kinetic behaviour of the reaction, it gives a quick and easy view of which systems can obviously provide significant amounts of the desired adducts within an acceptable time. This preliminary screening (data shown in ESI†) allowed us to identify a few systems worthy of further investigation: water, ethanol, isopropanol, methyl-THF (MeTHF) and tetrahydrofurfuryl-alcohol (THFA) (also referred to as hydroxymethyl-THF). For practical purification reasons, other solvents with higher boiling points were excluded. A stoichiometric amount of DABCO and 2 equivalents of ethyl acrylate were used in all cases to allow consistent comparisons. The results are shown in Table 1. An initial observation is that water alone can be used for this reaction, though the reaction is quite slow, resulting in a 33% isolated yield after 24 h (entry 1). The TLC and NMR of crude mixtures show that significant amounts of HMF remained unreacted and no other product was formed, apart from the intermediate betaine produced by the Michael addition of DABCO with ethyl acrylate. Interestingly, when mixtures of solvents were used, the isolated yields were significantly improved, reaching an acceptable 70% or more within 24 h (entries 6, 7, 9). The example of MeTHF is specific, as there is no reaction in pure MeTHF whereas its mixture with water, though not completely homogeneous, led to acceptable yields of BH adducts within the same reaction time used for consistent comparison. A similar observation was made during the solvent screening (see ESI†) for diethyl succinate. Aqueous ethanol and aqueous tetrahydrofurfuryl alcohol (THFA) gave the best results. As regards the amount of promoter, decreasing to 0.8 equiv. was already clearly disadvantageous and resulted in lower yields (entry 10). This is consistent with reported observations showing that, although theoretically catalytic, a significant amount of the promoter is kept away from the desired process by the formation of a stable betaine resulting from hydrolysis of the Michael addition product of DABCO with ethyl acrylate.5d,e,g With respect to the acrylate, it was possible to decrease to 1.5 equivalents while maintaining acceptable yields, whereas a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was found to be less efficient (entries 11 and 12).
1 ratio was found to be less efficient (entries 11 and 12).
| Entry | Solvent | 2 | DABCO (equiv.) | 3 (%) |
|---|---|---|---|---|
a Binary mixtures are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol, mixtures; MeTHF: 2-methyl-tetrahydrofurane; THFA: tetrahydrofurfuryl alcohol. 1 vol, mixtures; MeTHF: 2-methyl-tetrahydrofurane; THFA: tetrahydrofurfuryl alcohol. |
||||
| 1 | H2O | 2 | 1 | 33 |
| 2 | EtOH | 2 | 1 | 15 |
| 3 | Isopropanol | 2 | 1 | 17 |
| 4 | MeTHF | 2 | 1 | 0 |
| 5 | THFA | 2 | 1 | 29 |
| 6 | EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2 | 1 | 75 |
| 7 | Isopropanol/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2 | 1 | 74 |
| 8 | MeTHF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2 | 1 | 50 |
| 9 | THFA/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2 | 1 | 72 |
| 10 | THFA/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2 | 0.8 | 60 |
| 11 | THFA/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.5 | 1 | 71 |
| 12 | THFA/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.0 | 1 | 40 |
In order to investigate further the importance of the ratio between water and the bio-based solvent, a short study, from the synthetic viewpoint, was performed by measuring the adduct/HMF ratio as a function of time, by NMR in the case of THFA. This revealed that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was the most efficient one but a 4
1 ratio was the most efficient one but a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water–THFA ratio was nearly as good, whilst a water-poor 1
1 water–THFA ratio was nearly as good, whilst a water-poor 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mixture was less efficient (Fig. 1). Our results confirm that addition of water is beneficial to the BH reaction of HMF, in agreement with previous studies regarding aqueous BH reactions, indicating that water might act by stabilizing the transition state or the intermediates (enolate, zwitterionic adduct) through intermolecular charge-dipole and/or hydrogen-bonding interactions.5,6 However, in this specific case of HMF, pure water does not provide the best result whereas binary systems show the best balance for accommodating all the parameters of the reaction.
4 mixture was less efficient (Fig. 1). Our results confirm that addition of water is beneficial to the BH reaction of HMF, in agreement with previous studies regarding aqueous BH reactions, indicating that water might act by stabilizing the transition state or the intermediates (enolate, zwitterionic adduct) through intermolecular charge-dipole and/or hydrogen-bonding interactions.5,6 However, in this specific case of HMF, pure water does not provide the best result whereas binary systems show the best balance for accommodating all the parameters of the reaction.
 | ||
| Fig. 1 Evolution of the adduct/HMF ratio ([3]/[1]+[3]), measured by 1H NMR, as a function of time for different ratios of water and tetrahydrofurfuryl alcohol (HTHFA). | ||
Numerous solvent parameters can influence the overall efficiency of the reaction: (i) the polarity, influencing the stabilization of intermediates, (ii) the miscibility with water, (iii) the effect on acidity and its influence on the proton abstraction in the rate determining step, (iv) the ability to modulate protic activation of the electron-withdrawing group of the Michael acceptor and of the aldehyde, (v) the hydrophobic effect and its influence on the indispensable Michael addition of the promoter onto the activated olefin, (vi) the competitive hydrolysis of ethyl acrylate, or the intermediate Michael adducts or the BH adduct itself. There is also the ability to act as a shuttle easing the proton transfer in this very same rate determining step, but a recent study by Plata and Singleton6i resulted in refuting this shuttle process to the profit of solvent mediated acid–base steps during the proton transfer from the aldol alcoholate to the enolate intermediate able to undergo the last step of the pathway, i.e. the elimination of the tertiary amine. This very same study also pointed out that this proton transfer step was, though the main rate-limiting step, not the only one, competing with the aldol C–C bond formation step, notably at low temperature.6i Indeed, complete discrimination between all parameters requires intense and complex kinetic and theoretical studies.
From the viewpoint of synthetic organic chemistry, our results show that, although the use of pure water would be preferable, addition of a co-solvent is indispensable for reaching acceptable yields in the BH transformation of HMF. Moreover, a water-rich mixture is preferable to a water-poor mixture. The favorable effect of water on the Michael addition is well documented,9,10 thus addition step (amine + DABCO) must be facilitated, and the zwitterionic intermediate stabilized. The aldol step is also known to be facilitated by water.10,11 However, undesired competitive hydrolysis to the betaine, which consumes both the acrylate and the promoter, can also occur.
When the water–solvent ratio parameter was investigated in previous studies on aqueous BH reactions using classical solvents such as THF, dioxane, DMF, etc., results were rather inconsistent. While 5% aqueous THF or 10% aqueous DMF was preferred for the proline catalyzed reaction of p-nitrobenzaldehyde with methyl vinylketone reported by Tomkinson and coworkers,5c another work5h by Vasconcellos demonstrated that 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 t-butanol–water or DMSO–water gave the best results depending on the Michael acceptor, either acrylonitrile or methylacrylate, close to the optimum 1
40 t-butanol–water or DMSO–water gave the best results depending on the Michael acceptor, either acrylonitrile or methylacrylate, close to the optimum 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dioxane–water found by Hu and coworkers in their study5d of the MBH reaction of p-nitrobenzaldehyde with methyl acrylate. In Coelho's work12 on the 6,7-dihydro-5H-pyrrolo[1,2-a]imidazole (DPI) catalyzed reaction of variously substituted aldehydes with cyclopentenone, the best yields were obtained using a 4
1 dioxane–water found by Hu and coworkers in their study5d of the MBH reaction of p-nitrobenzaldehyde with methyl acrylate. In Coelho's work12 on the 6,7-dihydro-5H-pyrrolo[1,2-a]imidazole (DPI) catalyzed reaction of variously substituted aldehydes with cyclopentenone, the best yields were obtained using a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water–THF mixture. This means that a delicate balance has to be found. Since the focus of our study is the HMF scaffold, this led us to investigate the influence of the starting aldehyde polarity, by comparing with the less polar furfural (4), missing the CH2OH substituent at C-5, or the more polar α-D-glucosyloxymethylfurfural (GMF, 6), in which the CH2OH bears a complete glucosyl moiety.
1 water–THF mixture. This means that a delicate balance has to be found. Since the focus of our study is the HMF scaffold, this led us to investigate the influence of the starting aldehyde polarity, by comparing with the less polar furfural (4), missing the CH2OH substituent at C-5, or the more polar α-D-glucosyloxymethylfurfural (GMF, 6), in which the CH2OH bears a complete glucosyl moiety.
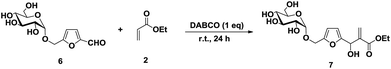
For furfural, readily available from pentoses,13 the formation of the adduct 5 (ref. 5b) proceeded in water or pure bio-based solvents, with better results in binary mixtures as observed for HMF (Table 2). When GMF, an interesting scaffold obtained by dehydration of the disaccharide isomaltulose14 was used, it was found that, again, bio-based solvents can be used to provide decent yields of BH adducts 7,8 however in this case, results were almost identical with or without water (Table 3). Compared to HMF, the 4 OH groups present in GMF and the BH intermediates and adducts apparently provide sufficient higher polarity and protic character for increasing the polarity and providing protic assistance in the process. This result shows that there is no generality in the optimal water-co-solvent ratio, being sensitive to numerous parameters, in particular the polarity and the hydroxyl content of the substrate.
| Entry | Solvent | 5a (%) |
|---|---|---|
a 2 equiv. of acrylate; binary mixtures are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol mixtures. 1 vol mixtures. |
||
| 1 | H2O | 45 |
| 2 | EtOH | 35 |
| 3 | HTHF | 33 |
| 4 | EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
90 |
| 5 | HTHF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 |
| Entry | Solvent | 7a (%) |
|---|---|---|
a All reactions were conducted using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of GMF 6a and ethyl acrylate 2a; Yields of isolated product. 2 molar ratio of GMF 6a and ethyl acrylate 2a; Yields of isolated product. |
||
| 1 | H2O | 35 |
| 2 | EtOH | 65 |
| 3 | THFA | 61 |
| 4 | EtOH/H2O | 50 |
| 5 | THFA/H2O | 60 |
Conclusion
Binary mixtures of tetrahydrofurfuryl alcohol (THFA) with water, or ethanol with water, appear to be efficient media for the BH reaction of HMF or furfural with ethyl acrylate. Among several bio-based solvents, THFA, MeTHF, ethanol and isopropanol offer alternatives to classical media for the Baylis–Hillman reaction. These results widen the scope of potential solvents that can be used for the transformations of the biobased platform molecules of HMF and analogues. When the starting aldehyde is less polar, mixtures of these solvents with water are more appropriate, whereas for the more polar GMF, the reaction can undergo in pure bio-based solvents.Experimental section
HMF (126 mg, 1.0 mmol) was mixed with DABCO (112 mg, 1.0 mmol) and ethyl acrylate (200 mg, 2.0 mmol) in 2 mL of EtOH/H2O (1/1, v/v) under air. Upon completion (TLC), the reaction mixture was diluted with tert-butyl methyl ether (20 mL) and HCl (1 M, 2 mL), and neutralized with saturated NaHCO3 solution. The aqueous layer was separated and further extracted with tert-butyl methyl ether (2 × 20 mL). After drying the combined organic layers over NaSO4, followed by filtration and evaporation under reduced pressure, the crude mixture was purified by column chromatography eluting with CH2Cl2/Et2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the BH product (169 mg, 75%) as a colourless liquid. When higher boiling point solvents were used (such as THFA), the evaporation step prior to chromatography was continued by distillation under vacuum (0.1–1 mm Hg). Data for the new compound 3 are given below. General methods, a complete NMR study of the preliminary solvent screening, detailed procedures for the furfural and GMF BH reactions and the NMR spectra of all adducts are given in SI.
1) to give the BH product (169 mg, 75%) as a colourless liquid. When higher boiling point solvents were used (such as THFA), the evaporation step prior to chromatography was continued by distillation under vacuum (0.1–1 mm Hg). Data for the new compound 3 are given below. General methods, a complete NMR study of the preliminary solvent screening, detailed procedures for the furfural and GMF BH reactions and the NMR spectra of all adducts are given in SI.
Ethyl-2-[hydroxy-(5-hydroxymethyl-furan-2-yl)methyl] acrylate (3)
Colorless liquid, 1H NMR (300 MHz, MeOD) 1.24 (t, 3H, Ja = 6.9 Hz, Jb = 14.1 Hz, OCH2CH3), 4.10–4.25 (m, 2H, OCH2CH3), 4.48 (s, 2H, H-6), 5.59 (s, 1H, H-7), 6.06 (d, 1H, J = 1.2 Hz, H-9a), 6.14 (d, 1H, J = 3.0 Hz, H-3), 6.24 (d, 1H, J = 2.7 Hz, H-4), 6.37 (d, 1H, J = 0.9 Hz, H-9b); 13C NMR (75 MHz, MeOD) 14.4 (OCH2CH3), 57.4 (C-6), 61.9 (OCH2CH3), 66.4 (C-7), 108.8 (C-3), 109.1 (C-4), 125.7 (C-9), 142.6 (C-8), 155.8 (C-5), 156.1 (C-2), 167.2 (CO2Et). MS m/z (ESI) calculated for C11H14NaO5, [M + Na]+ 249.0733; found 249.0734.Acknowledgements
The authors thank the Ministère de l’Enseignement Supérieur et de la Recherche (MESR) and CNRS for their financial support, as well as the Chinese Scholarship Council (UT-INSA-CSC call) for the fellowship to JNT.Notes and references
- (a) A. P. Rauter, P. Vogel and Y. Queneau, Carbohydrates in Sustainable Development I, Topics in Current Chemistry, Springer, Germany, 2010, vol. 294 Search PubMed; (b) A. P. Rauter, P. Vogel and Y. Queneau, Carbohydrates in Sustainable Development II, Topics in Current Chemistry, Springer, Germany, 2011, vol. 295 Search PubMed; (c) M. Philippe, A. Cavezza, P. Pichaud, S. Trouille and M. Dalko-Csiba, Carbohydrate Chemistry, ed. A. P. Rauter, T. K. Lindhorst and Y. Queneau, RSC, Cambridge, 2014, vol. 40, p. 1 Search PubMed; (d) F. W. Lichtenthaler, Carbohydrate as Organic Raw Materials, Wiley-VCH, Weinheim, 1991 Search PubMed; (e) G. Descotes, Carbohydrate as Organic Raw Materials, Wiley-VCH, Weinheim, 1993, vol. 2 Search PubMed; (f) H. van Bekkum, H. Röper and A. G. J. Voragen, Carbohydrate as Organic Raw Materials, Wiley-VCH, Weinheim, 1996, vol. 3 Search PubMed; (g) W. Praznik and H. Huber, Carbohydrate as Organic Raw Materials. WUV-Univ, Vienna, 1998, vol. 4 Search PubMed.
- (a) T. Werpy and G. Petersen, Top value added chemicals from biomass volume I-results of screening for potential candidates from sugars and synthesis gas. NREL/TP-510–35523, National Renewable Energy Laboratory, Golden, CO, 2004 Search PubMed; (b) J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC; (c) P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538 RSC; (d) A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411 CrossRef CAS PubMed; (e) M. J. Climent, A. Corma and S. Iborra, Green Chem., 2011, 13, 520 RSC; (f) R. A. Sheldon, Green Chem., 2014, 16, 950 RSC; (g) M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827 CrossRef CAS PubMed; (h) A. J. J. Straathof, Chem. Rev., 2014, 114, 1871 CrossRef CAS PubMed.
- (a) R.-J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499 CrossRef CAS PubMed; (b) F. W. Lichtenthaler, Acc. Chem. Res., 2002, 35, 728 CrossRef CAS PubMed; (c) T. Wang, M. W. Noltea and B. H. Shanks, Green Chem., 2014, 16, 548 RSC; (d) A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754 RSC.
- (a) A. B. Baylis and M. E. D. Hillman, Ger. Offen, 1972, 2, 113 (Chem. Abstr, 1972, 77, 34174q) Search PubMedM. E. D. Hillman and A. B. Baylis, U.S. Pat., 3, 743, 669, 1973 (b) K. Morita, Z. Suzuki and H. Hirose, Bull. Chem. Soc. Jpn., 1968, 41, 2815 CrossRef CAS; (c) S. E. Drewes and G. H. P. Roo, Tetrahedron, 1988, 44, 4653 CrossRef CAS; (d) D. Basavaiah, P. D. Rao and R. S. Hyma, Tetrahedron, 1996, 52, 8001 CrossRef CAS; (e) D. Basavaiah, A. J. Rao and T. Satyanarayana, Chem. Rev., 2003, 103, 811–891 CrossRef CAS PubMed; (f) V. Declerck, J. Martinez and F. Lamaty, Chem. Rev., 2009, 109, 1–48 CrossRef CAS PubMed; (g) D. Basavaiah, B. S. Reddy and S. S. Badsara, Chem. Rev., 2010, 110, 5447 CrossRef CAS PubMed; (h) Y. Wei and M. Shi, Chem. Rev., 2013, 113, 6659–6690 CrossRef CAS PubMed.
- (a) J. Auge, N. Lubin and A. Lubineau, Tetrahedron Lett., 1994, 35, 7947 CrossRef CAS; (b) D. Basavaiah, M. Krishnamacharyulu and A. J. Rao, Synth. Commun., 2000, 30, 2061 CrossRef CAS PubMed; (c) H. J. Davies, A. M. Ruda and N. C. O. Tomkinson, Tetrahedron Lett., 2007, 48, 1461 CrossRef CAS PubMed; (d) C. Yu, B. Liu and L. Hu, J. Org. Chem., 2001, 66, 5413 CrossRef CAS PubMed; (e) C. Yu and L. Hu, J. Org. Chem., 2002, 67, 219 CrossRef CAS PubMed; (f) V. K. Aggarwal, D. K. Dean, A. Mereu and R. Williams, J. Org. Chem., 2002, 67, 510 CrossRef CAS PubMed; (g) J. Cai, Z. Zhou, G. Zhao and C. Tang, Org. Lett., 2002, 4, 4723 CrossRef CAS PubMed; (h) R. O. M. A. de Souza, V. L. P. Pereira, P. M. Esteves and M. L. A. A. Vasconcellos, Tetrahedron Lett., 2008, 49, 5902 CrossRef CAS PubMed; (i) Y. Song, H. Ke, N. Wang, L. Wang and G. Zou, Tetrahedron, 2009, 65, 9086 CrossRef CAS PubMed; (j) A. V. Subrahmanyam, S. Thayumanavan and G. W. Huber, ChemSusChem, 2010, 3, 1158 CrossRef CAS PubMed.
- (a) L. S. Santos, C. H. Pavam, W. P. Almeida, F. Coelho and M. N. Eberlin, Angew. Chem., Int. Ed., 2004, 43, 4330 CrossRef CAS PubMed; (b) K. E. Price, S. J. Broadwater, H. M. Jung and D. T. McQuade, Org. Lett., 2005, 7, 147 CrossRef CAS PubMed; (c) K. E. Price, S. J. Broadwater, B. J. Walker and D. T. McQuade, J. Org. Chem., 2005, 70, 3980 CrossRef CAS PubMed; (d) V. K. Aggarwal, S. Y. Fulford and G. C. Lloyd-Jones, Angew. Chem., Int. Ed., 2005, 44, 1706 CrossRef CAS PubMed; (e) R. Robiette, V. K. Aggarwal and J. N. Harvey, J. Am. Chem. Soc., 2007, 129, 15513 CrossRef CAS PubMed; (f) G. W. Amarante, H. M. S. Milagre, B. G. Vaz, B. R. V. Ferreira, M. N. Eberlin and F. Coelho, J. Org. Chem., 2009, 74, 3031 CrossRef CAS PubMed; (g) D. Roy, C. Patel and R. B. Sunoj, J. Org. Chem., 2009, 74, 6936 CrossRef CAS PubMed; (h) D. Cantillo and C. O. Kappe, J. Org. Chem., 2010, 75, 8615 CrossRef CAS PubMed; (i) R. E. Plata and D. A. Singleton, J. Am. Chem. Soc., 2015, 137, 3811 CrossRef CAS PubMed.
- (a) C. K. Z. Andrade and L. M. Alves, Curr. Org. Chem., 2005, 9, 195 CrossRef CAS; (b) R. A. Sheldon, Green Chem., 2005, 7, 267 RSC; (c) I. T. Horváth, Green Chem., 2008, 10, 1024 RSC; (d) M. J. Hernáiz, A. R. Alcántara, J. I. García and J. V. Sinisterra, Chem.–Eur. J, 2010, 16, 9422 CrossRef PubMed; (e) H.-P. Meyer, Org. Process Res. Dev., 2011, 15, 180 CrossRef CAS; (f) Y. Gu and F. Jérôme, Green Chem., 2010, 12, 1127 RSC; (g) Y. Gu, Green Chem., 2012, 14, 2091 RSC; (h) Y. Gu and F. Jérôme, Chem. Soc. Rev., 2013, 42, 9550 RSC.
- J.-N. Tan, M. Ahmar and Y. Queneau, RSC. Adv., 2013, 3, 17649 RSC.
- (a) A. Lubineau and J. Augé, Tetrahedron Lett., 1992, 33, 8073 CrossRef CAS; (b) A. Lubineau, G. Bouchain and Y. Queneau, J. Chem. Soc., Perkin Trans. 1, 1995, 2433 RSC; (c) G. Giorgi, P. Lopez-Alvarado, S. Miranda, J. Rodriguez and J. C. Menendez, Eur. J. Org. Chem., 2013, 1327 CrossRef CAS PubMed.
- For review on water mediated chemical transforamtions, see: C. J. Li, Chem. Rev., 1993, 93, 2023 CrossRef CAS; A. Lubineau, J. Augé and Y. Queneau, Synthesis, 1994, 741 CrossRef; C. J. Li, Chem. Rev., 2005, 105, 3095 CrossRef PubMed.
- (a) T. Kitanosomo and S. Kobayashi, Adv. Synth. Catal., 2013, 355, 3095 CrossRef PubMed; (b) A. Lubineau, J. Org. Chem., 1986, 51, 2142 CrossRef CAS; (c) A. Lubineau and E. Meyer, Tetrahedron, 1988, 44, 6065 CrossRef CAS.
- J. C. Gomes, M. T. Rodrigues Jr, A. Moyano and F. Coelho, Eur. J. Org. Chem., 2012, 6861 CrossRef CAS PubMed.
- (a) G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044 CrossRef CAS PubMed; (b) R. Sahu and P. L. Dhepe, ChemSusChem, 2012, 5, 751 CrossRef CAS PubMed; (c) X. Hu, C. Lievens and C. Z. Li, ChemSusChem, 2012, 5, 1427 CrossRef CAS PubMed.
- (a) F. W. Lichtenthaler and S. Peters, C. R. Chim., 2004, 7, 65 CrossRef CAS PubMed; (b) T. Hanemann, E. Schumacher, W. Haase and F. W. Lichtenthaler, Liq. Cryst., 1997, 22, 47 CrossRef CAS PubMed; (c) F. W. Lichtenthaler, D. Martin, T. Weber and H. Schiweck, Liebigs Ann. Chem., 1993, 967 CrossRef CAS PubMed; (d) F. W. Lichtenthaler, D. Martin, T. Weber and H. Schiweck, D.E. Pat. 3,936,522, 1989; (e) C. Ruβ, C. Luff, A. H. Begli and B. Koenig, Synth. Commun., 2012, 42, 3112 CrossRef PubMed; (f) J.-N. Tan, M. Ahmar and Y. Queneau, Curr. Org. Chem., 2014, 18, 1768 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14554f |
| This journal is © The Royal Society of Chemistry 2015 |