4-Dodecylbenzenesulfonic acid (DBSA) promoted solvent-free diversity-oriented synthesis of primary carbamates, S-thiocarbamates and ureas†
Abstract
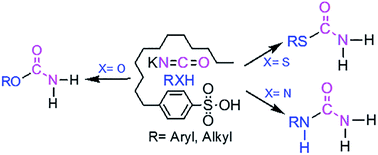
A simple and highly efficient solvent-free method for the conversion of alcohols, phenols, thiols and amines to primary carbamates, S-thiocarbamates and ureas in the presence of 4-dodecylbenzenesulfonic acid (DBSA) as a cheap and green Brønsted acid reagent has been described. All products were obtained in good to excellent yields and characterized using FT-IR, 1H- and 13C-NMR, MS and CHNS techniques.


 Please wait while we load your content...
Please wait while we load your content...