Controllable synthesis of graphene using novel aromatic 1,3,5-triethynylbenzene molecules on Rh(111)†
Abstract
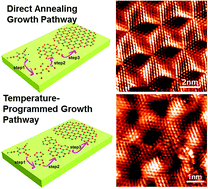
Selecting distinctive carbon precursors can mediate graphene synthesis towards versatile targets, for example, using C60 to produce graphene quantum dots or hexabromobenzene to achieve graphene at rather low temperature. Herein, 1,3,5-triethynylbenzene (TEB) is selected for the first time as the carbon precursor for graphene synthesis on Rh(111). Considering the characteristic π–d orbital hybridization between TEB and Rh(111), room-temperature adsorption followed by a temperature-programmed annealing or direct annealing to target growth temperature under ultrahigh vacuum conditions is designed to be the two synthesis pathways. In the former growth pathway, the benzene ring of the TEB unit is expected to be maintained throughout the whole annealing process, leading to a high yield of graphene at relatively low temperature as compared with existing synthesis via gaseous precursors. Several-molecule oligomers and graphene nanoclusters are detected to be the crucial intermediates through stepwise annealing (150 °C and 430 °C), as evidenced by scanning tunneling microscopy (STM) characterizations. Interestingly, graphene synthesized via the latter pathway usually possesses fewer domain boundaries and defects than the former one, probably due to sufficient diffusion and rearrangement of carbon precursors. Briefly, this work should contribute greatly to understanding the growth intermediates and the synthesis of high-quality graphene using large aromatic precursor molecules.

 Please wait while we load your content...
Please wait while we load your content...