Open Access Article
Open Access Article1-Hexanol triggered structural characterization of the worm-like micelle to vesicle transitions in cetyltrimethylammonium tosylate solutions
Vijay
Patel
a,
Debes
Ray
 b,
Kulbir
Singh
c,
Ludmila
Abezgauz
d,
Gerrard
Marangoni
c,
Vinod K.
Aswal
b and
P.
Bahadur
*e
b,
Kulbir
Singh
c,
Ludmila
Abezgauz
d,
Gerrard
Marangoni
c,
Vinod K.
Aswal
b and
P.
Bahadur
*e
aDepartment of Chemistry, Vidhyadeep Institute of Science, Anita (Kim), Olpad, Surat-394110, India. E-mail: vijaypatel4686@gmail.com
bSolid State Physics Division, Bhabha Atomic Research Centre, Mumbai-400085, India. E-mail: debes@barc.gov.in; vkaswal@barc.gov.in
cDepartment of Chemistry, St. Francis Xavier University, Antigonish NS B2G 2W5, Canada. E-mail: chembeer@gmail.com; gmarango@stfx.ca
dDepartment of Biotechnology and Food Engineering, Technion-Israel Institute of Technology, Haifa, 32000, Israel. E-mail: ludaa@technion.ac.il
eDepartment of Chemistry, Veer Narmad South Gujarat University, Surat-395007, India. E-mail: pbahadur2002@yahoo.com; pbahadur2002@gmail.com; Tel: +91-9879132125
First published on 7th October 2015
Abstract
The cationic surfactant cetyltrimethylammonium tosylate (CTAT) forms highly viscous/viscoelastic solutions and worm-like micelles at relatively low concentrations. The effect of 1-alkanols with short to long alkyl chains viz. ethanol, 1-butanol, 1-hexanol and 1-octanol on CTAT micelles was examined. In particular, a detailed study on the effect of 1-hexanol was carried out by viscosity, cryogenic transmission electron microscopy (cryo-TEM), nuclear magnetic resonance (NMR) and small-angle neutron scattering (SANS) to observe microstructural changes in CTAT micelles. 1-Hexanol displays a distinct interaction with CTAT micelles strongly dependent on CTAT concentration. Up to a certain critical CTAT concentration, 1-hexanol molecules penetrate into the micelles and show growth. Characterization by direct cryo-TEM imaging implies that upon progressive addition of 1-hexanol, worm-like CTAT micelles first grow and finally transform into vesicles. The course of vesicle formation was followed by SANS measurements. The site of 1-hexanol in the micelles close to the palisade layer was evaluated using 2D NMR. This study devotes a fundamental knowledge for controlling the shape and size of worm-like micelles that find many industrial applications particularly in personal- and home-care products.
1. Introduction
In aqueous solution, quaternary ammonium salt based cationic surfactants viz. cetyltrimethylammonium bromide (CTAB) form rod- or worm-like micelles, much above the critical micelle concentration (CMC) or in the presence of salts such as NaCl, NaBr, NaNO3 and NaClO3 even at concentrations close to CMC.1–3 The presence of salt screens the electrostatic repulsion between the charged head groups and facilitates micellar growth.4,5 However, cationic surfactants with strongly bound hydrophobic counterions such as cetyltrimethylammonium tosylate (p-toluenesulfonate) (CTAT) can form worm-like micelles even in the absence of salt and at a much lower concentration.6–8 The CMC of CTAT is relatively low (0.3 mM), rod-like micelles form at about 15 mM and transform into viscoelastic gels around 70 mM.9–13 The solution behavior of CTAT is comparable to that of high molecular weight polymer solutions where molecules entangle above their coil-overlap concentration.14,15 Here, tosylate counterions insert between the head groups in micelles and promote micellar growth to worm-like micelles and viscoelasticity. Worm-like micelles are sometimes called living polymers due to the fact that they are in dynamic equilibrium with monomers.16 Such micellar systems exhibit peculiar rheological properties and find applications in diverse fields ranging from personal care products to drilling fluids for enhanced oil recovery, drag reduction, as templates for material synthesis, cosmetics, paints, fracturing fluids in oil production and are also ideal candidates for the study of complex flow phenomenon.17–22The rheological properties of aqueous CTAT solutions can be influenced by the presence of anionic surfactant,5,15 salts,23–25 polymers12,26–28 and by temperature.25,29 Koehler et al.5 studied the effect of sodium dodecylbenzene sulfonate (SDBS) on CTAT solution and observed growth of worm-like micelles, though at higher concentrations strong mutual interactions between the oppositely charged surfactants facilitated a transition from linear micelles to branched micellar network of low viscosity. Rojas et al.30 studied the effect of hydroxyethyl cellulose (HEC) and hydrophobically modified hydroxyethyl celluloses (HMHEC) with different molecular weights on the shear viscosity of CTAT solution. Their results show that the shear viscosity of HEC/CTAT solutions is higher than the viscosities of the surfactant or polymer solutions at the same concentrations, but are lower compared with the HMHEC/CTAT system. In addition, surface tension measurements manifest complex formation between CTAT and HMHEC but not for CTAT/HEC system.
In our previous study, we have examined the effect of Pluronic® F87, a hydrophilic nonmicellar PEO–PPO–PEO triblock copolymer, on worm-like micelles of CTAT.28 In the presence of F87, a sharp decrease in the CTAT solution viscosity was due to transformation of worm-like micelles into smaller micelles. Here, PPO segments of F87 inserts towards hydrophobic core of CTAT micelles. The PPO moieties contain significant amount of water and PEO is heavily hydrated and this results in increase in hydrophilicity. These additives are often used to modify the properties of surfactants solutions, allowing them to be utilized in diverse applications such as lubrication, oil-wetting cleaners, pharmaceuticals, cosmetics, food and detergent formulations, which require the use of surfactants in a water-poor medium.31–33
Short/medium/long chain alcohols have been of interest due their important role as co-solvents/co-surfactants in microemulsions. It is generally noticed that short chain alcohols (C1–C3) remain in the aqueous bulk phase, medium chain alcohols (C4–C6) partition between the micellar and bulk phases and long chain alcohols (C7 and above) are solubilized within the micelle with polar hydroxyl group protruding towards the micelle surface and inducing the microstructural changes in the system. Bahadur and coworkers34–41 have extensively studied the effect of different alcohols on aqueous surfactant solutions. 1-Hexanol is a weakly amphiphilic alcohol with low aqueous solubility, which is known to penetrate into micelles and promote their growth. There have been other reports on the effect of 1-hexanol on several surfactants viz. cationic,34 gemini,36,42 sugar surfactant,43 Triton X-100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 and Pluronics® with varying hydrophobicity.38–41 Few studies demonstrate that addition of hydrophobic alcohol leads to growth of worm-like micelle44,45 followed by vesicle formation.46–48 Sreejith et al.49 observed 1-octanol induced a micelle-to-vesicle transition in CTAB/KBr system that was dependent on the alcohol concentration and temperature. These vesicular structures are extensively studied due to their prime importance in numerous applications.49–51 Generally, vesicles are formed by interaction of two oppositely charged surfactants. The formation of vesicular structures has also been reported for CTAT micelles in the presence of anionic surfactant SDBS.52,53 Kaler et al.52 demonstrated the spontaneous formation of equilibrium vesicle of CTAT in the presence of SDBS and it was proposed that vesicle size can readily be tuned by changing the composition of both the surfactants. However, a study involving additive induced vesicle formation in salt-free CTAT in aqueous solution has not been attempted yet.
37 and Pluronics® with varying hydrophobicity.38–41 Few studies demonstrate that addition of hydrophobic alcohol leads to growth of worm-like micelle44,45 followed by vesicle formation.46–48 Sreejith et al.49 observed 1-octanol induced a micelle-to-vesicle transition in CTAB/KBr system that was dependent on the alcohol concentration and temperature. These vesicular structures are extensively studied due to their prime importance in numerous applications.49–51 Generally, vesicles are formed by interaction of two oppositely charged surfactants. The formation of vesicular structures has also been reported for CTAT micelles in the presence of anionic surfactant SDBS.52,53 Kaler et al.52 demonstrated the spontaneous formation of equilibrium vesicle of CTAT in the presence of SDBS and it was proposed that vesicle size can readily be tuned by changing the composition of both the surfactants. However, a study involving additive induced vesicle formation in salt-free CTAT in aqueous solution has not been attempted yet.
Herein, we report on the aqueous solution behavior of CTAT in the presence of 1-hexanol accompanied by worm-like micelles-to-vesicles transition. Microstructural changes are function of 1-hexanol and CTAT concentrations and are scrutinized by viscosity, cryo-TEM and SANS. Ethanol, 1-butanol and 1-octanol were used to compare the viscosity behavior of CTAT. Location of 1-hexanol in micelles was elucidated from NOESY. The present study offers a simple way of getting desired morphology of worm-like micelles easily tuned up by 1-hexanol.
2. Experimental
2.1 Materials
Cetyltrimethylammonium tosylate (CTAT) was purchased from Sigma Aldrich and used without further purification. Ethanol, 1-butanol, 1-hexanol and 1-octanol were obtained from Spectrochem, India and used as received. Deionized water from a Millipore Milli-Q system was used to prepare samples for viscosity and cryo-TEM measurements. The solutions for SANS and NMR were prepared in D2O (99.9% pure).2.2 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(θ/2)/λ, where θ is scattering angle) range of 0.017 to 0.35 Å−1. Sample temperature was kept constant at 30 °C for all measurements. The measured SANS data were corrected for the background, empty cell contribution and the transmission and normalized to absolute cross-sectional unit using standard protocols.
sin(θ/2)/λ, where θ is scattering angle) range of 0.017 to 0.35 Å−1. Sample temperature was kept constant at 30 °C for all measurements. The measured SANS data were corrected for the background, empty cell contribution and the transmission and normalized to absolute cross-sectional unit using standard protocols.
2.3 Results and discussion
 | ||
| Fig. 1 Chemical structures and proton labeling of (a) cetyltrimethylammonium tosylate (b) 1-hexanol. | ||
In earlier studies, such micellar growth has been observed in the presence of an oppositely charged surfactant or when inorganic salt was added to surfactant solutions. For ionic surfactants, the electrostatic repulsions between the polar groups are strong which leads to a large effective head group and favor the formation of relatively smaller micelles. However, the added salt or oppositely charged amphiphile screens net surface charge and thus decreases the effective area per head group and favors micellar growth/transition.4,5 In the present case, due to hydrophobic tosylate counterion, worm-like (linear) micelles exist even in the absence of salt, which results in a high solution viscosity. These propose that the viscosity of CTAT solution in the presence of alcohols is influenced by an additional factor.
The effect of alcohols chain length on the viscosity of cationic surfactant can be explained by their ability to partition between the aqueous bulk phase and the pseudomicellar phase. The longer chain (more hydrophobic) alcohols are efficiently solubilized in the micelle compared to shorter chain (more hydrophilic) alcohols which mostly affect solvent properties. As indicated from CMC values of surfactants in presence of alcohols, hydrophobic alcohols cause a larger decrease in the CMC than hydrophilic ones.36 Alternatively, more hydrophobic alcohols have higher partition coefficient and they get solubilized in surfactant micelles at much lower concentrations than the shorter chain alcohols.58
It is well documented that short chain hydrophilic alcohols (like ethanol) behave as water structure breakers (co-solvents) and affect the micelle stability by altering the water dielectric constant.36 Their presence makes water (solvent) more affable for the hydrocarbon portion of the surfactant, resulting in increasing destruction of the micelles.59 Based on this explanation, the trend observed in the viscosity of CTAT solutions as a function of ethanol concentration can be understood. At 15 mM CTAT micelles are already large; small addition of ethanol transforms these micelles to a less compact state (i.e., micelle size increases), which is reflected as an increase in the viscosity. Above 0.6% of added ethanol, the large and less compact micelles slowly convert into smaller micelles, which is reflected in the drop in the viscosity. In the present study, the concentration of ethanol used is below its aqueous solubility so there is not any possibility of its penetration inside CTAT micelles which can lead to any morphological changes. Due to much lower dielectric constant of ethanol, its presence weakens the hydrophobic effect of CTAT and hence energy of system required for vesicle formation is not attained.60 So it can be inferred that ethanol is not capable of forming vesicle from CTAT. It can also be correlated with lower value of octanol–water partition co-efficient (Po/w) ∼ 0.49 for ethanol which opposes its penetration inside CTAT micelles.36 There is no report on ethanol induced micellar growth which leads to formation of branched worm-like micelles or vesicles formation of single surfactant at higher concentration so it was safely concluded that disintegration of micelles to smaller size is responsible for lowering of solution viscosity at higher ethanol concentration. As compared to ethanol, in the presence of 1-butanol, the peak viscosity appears at relatively lower concentration. Since the hydrophobicity of the micellar interior is high (due to insertion of the tosylate counterion) and 1-butanol is relatively more hydrophobic and has higher Po/w compared to ethanol, it partitions between the micellar and the bulk phases at a lower concentrations. Such a partition increases micellar size and stimulates slight increase in the solution viscosity. However, with increase in 1-butanol concentrations, the solubility of the nonpolar tail of CTAT is increased; hence, the hydrophobic interactions between the surfactant tails are reduced which will affect the amount of surfactant assembling into micelles. This will lead to a decrease in the micellar size and consequently a decrease in the viscosity is observed. At higher concentration, 1-butanol also behaves as co-solvent similar to ethanol and does not contribute to vesicular formation at all concentrations.34 So it was expected that worm-like CTAT micelles are transformed to smaller micelles at higher 1-butanol concentrations responsible for lower solution viscosity. However, 1-hexanol with higher Po/w (∼107), intercalates between the head group of CTAT micelles at lower concentrations and reduces surface charge density and promotes micellar growth, which is reflected in higher solution viscosity. It is reported that 1-hexanol is capable of inducing the growth of wormlike micelles that can be transformed into vesicles at higher concentrations.42 Similarly, in the present study, a dramatic loss in solution viscosity at higher [1-hexanol] is observed which suggests that such a transition is accompanied by variety of microstructures. Such observations prompted us to study the effect of 1-hexanol on CTAT solution in detail. A detailed study on formation of vesicles from CTAB micelles in the presence of KBr has been reported for 1-octanol and it was concluded that sudden drop in viscosity after maximum is due to formation of vesicles.49 It is well reported that 1-octanol increases the aggregation number by forming mixed micelles.45,49 It is obvious from above observations that 1-hexanol and 1-octanol are capable of forming vesicles from single surfactant solution while ethanol and 1-butanol are not. In the present study, due to more hydrophobic nature of 1-octanol, it intercalates between CTAT charged head groups and leads to a large increase in viscosity than 1-hexanol at much lower concentration. Consequently, viscosity maximum appears at 0.1% for 1-octanol. So it can be concluded that extent of penetration of 1-alkanols which contributes to higher solution viscosity is greater for 1-alkanols having alkyl chain length higher than 1-butanol so viscosity maximum is shifted to lower 1-alkanol concentration and the magnitude of increase in viscosity follows the order 1-octanol > 1-hexanol > 1-butanol > ethanol.
The microstructures formed have been found to be the function of CTAT concentration as well. In this context, viscosity measurements were performed by varying CTAT concentration in the presence of 1-hexanol; the results are shown in Fig. 3. The critical rod concentration (CRC) for CTAT is 15 mM.9,10 Below the CRC, the addition of 1-hexanol does not lead to large increase in viscosity and we get a good viscosity graph at 20 mM CTAT expecting variety of microstructures formed so we performed detailed study this concentration. Addition of 1-hexanol to 20 mM CTAT solution has a striking effect on the solution viscosity showing a maximum at 0.25% 1-hexanol. With increase in [CTAT], the maximum shifts to lower 1-hexanol concentration.49 In 30 mM CTAT, the viscosity directly decreases without showing any maximum. This may be due to a more compact packing of micelles at higher [CTAT] which does not allow 1-hexanol molecules to penetrate.
Such a dramatic changes in viscosity is a clear indication of transition in the micellar architecture in the system. Agarwal et al.61 have examined the effect of phenolic compounds on CTAB micellar solutions; the nonpolar moiety of these solutes interacts with the surfactant tail and the polar phenolic group with water. Phenol and cresol induced a sphere-to-rod-to worm-like micelles while 4-ethyl phenol and 4-butyl phenol showed a sphere to worm-like to multilamellar vesicle transitions. Zhang et al.62 reported that benzyl alcohol is solubilized in interfacial region and promotes growth of wormlike salted CTAB micelles while their transformation into small rods and then spherical ones at higher level of solubilization is responsible for peak in viscosity. In literature, a concept of vesicle formation has been claimed for peak in viscosity.42,49 1-Hexanol induced micelle-to-vesicle transition via formation of elongated micelles has been reported for the cationic gemini surfactant ethyl-α,ω-bis(dodecyldimethylammonium bromide).42 A study on the effect of alkanols ranging from C3 to C9 on sucrose monohexadecanoate (sugar surfactant) showing a similar micellar transition has also been reported.63 These results demonstrate that the length of worm-like micelles can easily be controlled by the addition of alkanols.
The focus of the present study is on the influence of 1-hexanol on CTAT worm-like micelles and formation of vesicular structures; a detailed study was performed at 20 mM surfactant concentration. At this concentration the solution contains worm-like micelles that are able to take up some 1-hexanol, hence at a lower 1-hexanol concentration an increase in the viscosity is observed followed by a sudden drop in viscosity at higher 1-hexanol concentration.42 Such a dramatical change in the viscosity is attributed to morphological changes in the system and is further quantified by cryo-TEM and SANS.
 | ||
| Fig. 5 SANS data of 20 mM CTAT as a function of 1-hexanol concentration at 30 °C. The data have been shifted vertically for clarity. Inset shows the data as measured. | ||
In the presence of 0.25% 1-hexanol, the scattering intensity of CTAT solution as well as the slope at low-Q remain almost same. This is consistent with the formation of elongated worm-like CTAT micelles at 0.25% 1-hexanol was proposed by cryo-TEM (Fig. 4b). On the basis of cryo-TEM and the observed increase in the viscosity and cryo-TEM image, it is evident that worm-like CTAT micelles elongate with increasing concentration of 1-hexanol. For worm-like micellar model, the chain of contour length L (total length) can be described a chain of some number of locally stiff segments (length lp). The persistence length lp is the length along the cylinder over which the flexible cylinder can be considered as a rigid rod. The Kuhn length (b) used in the model is also used to describe the stiffness of a chain, and is simply b = 2lp. At 0.5% 1-hexanol a sharp increase in the scattering intensity in the low-Q region suggests of the presence of larger structures. It was debated that the formation of branched worm-like micelles and/or vesicles is responsible for the sudden drop in the viscosity (cryo-TEM Fig. 4c). SANS plot showing slope of (−2) on log–log scale is a clear indication of the presence of vesicular structures at this concentration.71,72 There is dramatic decrease (about a factor of 5) in the magnitude of scattering with 1.5% 1-hexanol, suggesting some kind of phase separation because of the formation of much larger structures (perhaps multilamellar vesicles). These large structures could not be measured prior to their phase separation as the time for the SANS measurements (∼5 h) is much larger than over the period they exists. The fitted structural parameters from SANS data are shown in Table 1. The systems consist of worm-like micelles mostly upto 0.25% 1-hexanol. Unilamellar vesicles coexist with the worm-like micelles at 0.5% 1-hexanol. At further higher concentrations of 1-hexanol, the system also shows phase separation (multilamellar vesicles). The location of 1-hexanol is a deciding factor to induce such microstructural changes in CTAT micelles which is further evaluated using NMR spectroscopy.
| [1-Hexanol] (%) | Cross-sectional radius of worm-like micellesaR (Å) | Vesicle thickness t (Å) | Shape |
|---|---|---|---|
| a Worm-like micelles: Kuhn length (b) > 2π/Qmin ∼ 430 Å and contour length (L) ≫ b. | |||
| 0 | 17.6 ± 0.3 | — | Worm-like |
| 0.1 | 17.5 ± 0.3 | — | Worm-like |
| 0.25 | 17.5 ± 0.3 | — | Worm-like |
| 0.5 | 17.3 ± 0.3 | 54.6 ± 0.8 | Worm-like + unilamellar vesicles |
| 1.5 | 17.3 ± 0.6 | 55.2 ± 0.8 | Worm-like + unilamellar vesicles + multilamellar vesicles |
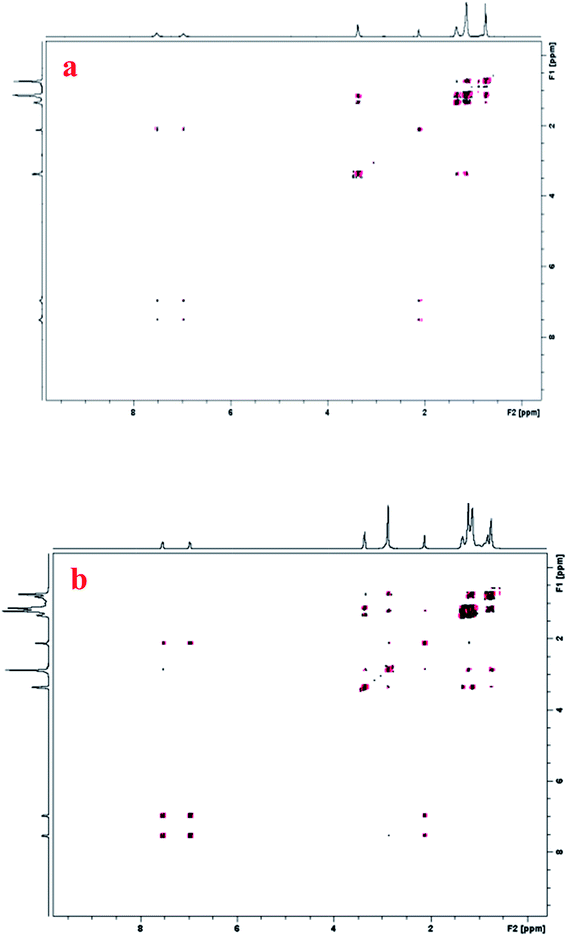
In the presence of 0.25% 1-hexanol, an intense NMR signals at ∼3.6, ∼1.2, 0.8 ppm corresponding to α, internal and terminal protons of 1-hexanol, respectively are observed. But at 0.5% the intensity of these signals is decreased clearly indicating that these protons are more shielded and experiences a more hydrophobic environment. It manifests penetration of 1-hexanol inside CTAT micelles at higher level of solubilization. At 0.25% 1-hexanol, intensity of head group protons remains unaffected but at 0.5% 1-hexanol it sharply increases. It reveals that these protons experience more hydrophilic environment possibly due to formation of few vesicles at 0.5% 1-hexanol which provide internal cavity made up of water (cryo-TEM Fig. 4c). In the presence of 1-hexanol, the intensities of the aromatic protons and C2 protons of CTAT are decreased, suggesting that they are more shielded and feel a hydrophobic environment probably due to the vicinity to the 1-hexanol hydrocarbon chain. It clearly manifests that penetrated 1-hexanol molecules reside below head group of CTAT micelle and are in close proximity of tosylate counterion/near the palisade layer and induces micellar growth. It is reflected in merging of NMR signals of internal chain protons clearly suggesting the strong interaction in this region of CTAT micelles.73,74 Similarly, terminal protons reveal broadening and merging showing interaction due to bending of CTAT hydrocarbon chain.72 From the NMR study it is concluded that 1-hexanol displays strong interactions with internal chain protons of CTAT depicting the location of 1-hexanol between micelle–water interface and palisade layer of CTAT micelle. To get better insight on sight of location and interaction of 1-hexanol molecules in CTAT micelles, NOESY experiments were performed.
The NOESY contour plot for the 20 mM CTAT in the presence of 0.25% 1-hexanol is presented in Fig. 7a. Intra- and inter-molecular cross peaks are observed in the spectra suggesting the strong interactions of 1-hexanol with CTAT protons. The closeness of protons is deciding factor for intensity of cross peaks; the intensity of cross peak is high if two protons are much closer. It is clear from Fig. 7a that there are no crosspeaks between head group protons of CTAT with α-proton of 1-hexanol which conclude that 1-hexanol do not interact with CTAT head group at 0.25% concentration. Weak intra-molecular crosspeaks are observed for aromatic protons. Crosspeaks are observed between C2 protons of CTAT and aromatic protons. Intense crosspeaks are observed between α-proton of 1-hexanol with β-protons and internal chain protons of CTAT which indicates the strong interaction in this region of CTAT micelles. Many overlapping crosspeaks are observed for internal and terminal chain protons of CTAT and 1-hexanol presenting strong interaction between them. The appearance of many crosspeaks in the NOESY spectra at 0.5% 1-hexanol clearly reveals that it exhibits concentration dependent interaction with CTAT micelles (Fig. 7b). A close inspection of NOESY spectra suggests that there is an intra-molecular crosspeak between head group protons, aromatic protons and aromatic protons with –CH3 protons (C2) of tosylate counterion of CTAT. Low intensed cross peaks are observed for head group protons and internal chain protons of CTAT. There is no cross peak between α-proton of 1-hexanol with C2 protons or aromatic proton of tosylate ion indicating that –OH group of 1-hexanol is not in palisade layer of CTAT micelles. A weak crosspeak is observed between C4 proton and head group protons of CTAT which may be due to penetration of tosylate counter ion inside micelle. A weak crosspeaks between α-proton of 1-hexanol with β-protons of CTAT were also noticed. It suggests that –OH group of 1-hexanol remains slightly inside the micelle, away from head group. Intense crosspeaks between α-proton and terminal chain protons of 1-hexanol with internal chain proton of CTAT were observed, suggesting strong interactions between them. A cross peak between C2 protons of CTAT and internal chain proton of 1-hexanol is also observed. It manifests the location of 1-hexanol close to the palisade layer and below the head group inside the CTAT micelle. An intense cross peak between α and terminal protons of 1-hexanol suggest intra-molecular interaction or bending of 1-hexanol chain. From the NOESY study, it is concluded that 1-hexanol resides near the palisade layer and below the head group, inside CTAT micelles. 1-Hexanol exhibits concentration dependent interactions with different region of CTAT micelles and accordingly alter micellar morphology.
3. Conclusions
The effect of n-alkanols on CTAT worm-like micelles was examined by viscosity, SANS, cryo-TEM, NMR and NOESY experiments. Studies show that 1-alkanols majorly influence CTAT micelles. Longer chain n-alkanols are more efficient in promoting micellar growth compared to shorter chain n-alkanols.The motivation of the present study was to examine 1-hexanol aided microstructural transitions of worm-like CTAT micelles. A typical peak in viscosity is ascribed to elongation of the worm-like micelles and co-existence of short rod-like micelles with other structures. With further increase in 1-hexanol concentration length of worm-like micelles is sufficient to allow branching and micellar segments sliding on each other. Branched worm-like micelles accompanied by few vesicular structures are formed which is reflected in a sudden drop in the viscosity. Further addition of 1-hexanol up to its maximum solubility leads to the formation of vesicular dispersions. A complete course of vesicles formation from worm-like CTAT micelles via elongated and branched worm-like micelles and short rod-like micelles was successfully monitored by cryo-TEM. It is well confirmed by SANS. NOESY experiments disclose that 1-hexanol resides close to palisade layer and below the head group inside CTAT micelles. Doping with 1-hexanol offers a simple and easy method of controlling the size and shape of CTAT micelles from worm-like to vesicles that find many industrial applications as well as applications in personal and home care products and in templated synthesis of mesoporous materials42,79–81 and can also be utilized for micellar-enhanced ultrafiltration process.
Acknowledgements
Authors thank to UGC New Delhi for financial assistance [Project No. 37-527/2009(SR)]. We thank Prof. Dganit Danino for the cryo-TEM analysis.References
- T. Imae, A. Abe and S. Ikeda, Viscosity behavior of semiflexible rodlike micelles of alkyltrimethylammonium halides in dilute and semidilute solutions, J. Phys. Chem., 1988, 92, 1548–1553 CrossRef CAS.
- K. Kuperkar, L. Abezgauz, K. Prasad and P. Bahadur, Formation and growth of micelles in dilute aqueous CTAB solutions in the presence of NaNO3 and NaClO3, J. Surfactants Deterg., 2010, 13, 293–303 CrossRef CAS.
- K. Kuperkar, L. Abezgauz, D. Danino, G. Verma, P. Hassan, V. Aswal, D. Varade and P. Bahadur, Viscoelastic micellar water/CTAB/NaNO3 solutions: rheology, SANS and cryo-TEM analysis, J. Colloid Interface Sci., 2008, 323, 403–409 CrossRef CAS PubMed.
- H. Rehage and H. Hoffmann, Viscoelastic surfactant solutions: model systems for rheological research, Mol. Phys., 1991, 74, 933–973 CrossRef CAS.
- R. D. Koehler, S. R. Raghavan and E. W. Kaler, Microstructure and dynamics of wormlike micellar solutions formed by mixing cationic and anionic surfactants, J. Phys. Chem. B, 2000, 104, 11035–11044 CrossRef CAS.
- V. Hartmann and R. Cressely, Shear thickening of an aqueous micellar solution of cetyltrimethylammonium bromide and sodium tosylate, J. Phys. II, 1997, 7, 1087–1098 CrossRef CAS.
- V. Hartmann and R. Cressely, Linear and non linear rheology of a wormlike micellar system in presence of sodium tosylate, Rheol. Acta, 1998, 37, 115–121 CrossRef CAS.
- G. Garg, P. Hassan and S. Kulshreshtha, Dynamic light scattering studies of rod-like micelles in dilute and semi-dilute regime, Colloids Surf., A, 2006, 275, 161–167 CrossRef CAS.
- J. Soltero, J. Puig and O. Manero, Rheology of the cetyltrimethylammonium tosilate–water system. 2. Linear viscoelastic regime, Langmuir, 1996, 12, 2654–2662 CrossRef CAS.
- J. Soltero, F. Bautista, J. Puig and O. Manero, Rheology of cetyltrimethylammonium p-toluenesulfonate–water system. 3. Nonlinear viscoelasticity, Langmuir, 1999, 15, 1604–1612 CrossRef CAS.
- S. B. Velegol and R. D. Tilton, Specific counterion effects on the competitive co-adsorption of polyelectrolytes and ionic surfactants, J. Colloid Interface Sci., 2002, 249, 282–289 CrossRef CAS PubMed.
- M. Truong and L. Walker, Controlling the shear-induced structural transition of rodlike micelles using nonionic polymer, Langmuir, 2000, 16, 7991–7998 CrossRef CAS.
- A. Müller, M. Torres and A. Saez, Effect of the flow field on the rheological behavior of aqueous cetyltrimethylammonium p-toluenesulfonate solutions, Langmuir, 2004, 20, 3838–3841 CrossRef.
- L. Mendoza, M. Rabelero, J. Escalante, E. Macías, A. Gonzalez-Alvarez, F. Bautista, J. Soltero and J. Puig, Rheological behavior of surfactant-based precursors of silica mesoporous materials, J. Colloid Interface Sci., 2008, 320, 290–297 CrossRef CAS PubMed.
- M. R. Rojas, A. J. Müller and A. E. Sáez, Effect of ionic environment on the rheology of wormlike micelle solutions of mixtures of surfactants with opposite charge, J. Colloid Interface Sci., 2010, 342, 103–109 CrossRef CAS PubMed.
- L. Wattebled and A. Laschewsky, Effects of organic salt additives on the behavior of dimeric (“Gemini”) surfactants in aqueous solution, Langmuir, 2007, 23, 10044–10052 CrossRef CAS PubMed.
- R. Bandyopadhyay and A. Sood, Effect of silica colloids on the rheology of viscoelastic gels formed by the surfactant cetyl trimethylammonium tosylate, J. Colloid Interface Sci., 2005, 283, 585–591 CrossRef CAS PubMed.
- B. A. Schubert, E. W. Kaler and N. J. Wagner, The microstructure and rheology of mixed cationic/anionic wormlike micelles, Langmuir, 2003, 19, 4079–4089 CrossRef CAS.
- M. Brigante and P. C. Schulz, Synthesis of mesoporous silicas in alkaline and acidic media using the systems cetyltrimethylammonium tosylate (CTAT)–Pluronic F127 triblock copolymer and CTAT–Pluronic F68 triblock copolymer as templates, J. Colloid Interface Sci., 2012, 369, 71–81 CrossRef CAS PubMed.
- J. Yang, Viscoelastic wormlike micelles and their applications, Curr. Opin. Colloid Interface Sci., 2002, 7, 276–281 CrossRef CAS.
- L. M. Walker, Rheology and structure of worm-like micelles, Curr. Opin. Colloid Interface Sci., 2001, 6, 451–456 CrossRef CAS.
- R. Zana and E. W. Kaler, Giant micelles: properties and applications, CRC Press, 2007, vol. 140, ch. 15–18 Search PubMed.
- J. Narayanan, C. Manohar, D. Langevin and W. Urbach, Growth of cetyltrimethylammonium tosylate Micelles: a frapp study, Langmuir, 1997, 13, 398–401 CrossRef CAS.
- M. F. Torres, J. M. González, M. R. Rojas, A. J. Müller, A. E. Sáez, D. Löf and K. Schillén, Effect of ionic strength on the rheological behavior of aqueous cetyltrimethylammonium p-toluene sulfonate solutions, J. Colloid Interface Sci., 2007, 307, 221–228 CrossRef CAS PubMed.
- E. Macías, F. Bautista, J. Pérez-López, P. Schulz, M. Gradzielski, O. Manero, J. Puig and J. Escalante, Effect of ionic strength on rheological behavior of polymer-like cetyltrimethylammonium tosylate micellar solutions, Soft Matter, 2011, 7, 2094–2102 RSC.
- J. C. Brackman and J. B. Engberts, Influence of polymers on the micellization of cetyltrimethylammonium salts, Langmuir, 1991, 7, 2097–2102 CrossRef CAS.
- T. Yoshida, R. Taribagil, M. A. Hillmyer and T. P. Lodge, Viscoelastic synergy in aqueous mixtures of wormlike micelles and model amphiphilic triblock copolymers, Macromolecules, 2007, 40, 1615–1623 CrossRef CAS.
- V. Patel, S. Chavda, V. K. Aswal and P. Bahadur, Effect of a hydrophilic PEO–PPO–PEO copolymer on cetyltrimethylammonium tosylate solutions in water, J. Surfactants Deterg., 2012, 15, 377–385 CrossRef CAS.
- R. Gamez-Corrales, J.-F. Berret, L. Walker and J. Oberdisse, Shear-thickening dilute surfactant solutions: equilibrium structure as studied by small-angle neutron scattering, Langmuir, 1999, 15, 6755–6763 CrossRef CAS.
- M. R. Rojas, A. Müller and A. Sáez, Synergistic effects in flows of mixtures of wormlike micelles and hydroxyethyl celluloses with or without hydrophobic modifications, J. Colloid Interface Sci., 2008, 322, 65–72 CrossRef CAS PubMed.
- S. Chavda, K. Singh, D. G. Marangoni, V. K. Aswal and P. Bahadur, Cationic micelles modulated in the presence of α,ω-alkanediols: a SANS, NMR and conductometric study, J. Surfactants Deterg., 2012, 15, 317–325 CrossRef CAS.
- K. Kuperkar, A. Patriati, E. Putra, K. Singh, D. Marangoni and P. Bahadur, Microstructural study of cetyltrimethylammonium bromide/1-butanol/salt/water system—SANS and 2D-NOESY analysis, Can. J. Chem., 2012, 90, 314–320 CrossRef CAS.
- S. Kulthe, N. Inamdar, Y. Choudhari, S. Shirolikar, L. Borde and V. Mourya, Mixed micelle formation with hydrophobic and hydrophilic Pluronic block copolymers: implications for controlled and targeted drug delivery, Colloids Surf., B, 2011, 88, 691–696 CrossRef CAS PubMed.
- K. C. Kuperkar, J. P. Mata and P. Bahadur, Effect of 1-alkanols/salt on the cationic surfactant micellar aqueous solutions—a dynamic light scattering study, Colloids Surf., A, 2011, 380, 60–65 CrossRef CAS.
- A. Desai, D. Varade, J. Mata, V. Aswal and P. Bahadur, Structural transitions of cetyltrimethylammonium bromide micelles in aqueous media: effect of additives, Colloids Surf., A, 2005, 259, 111–115 CrossRef CAS.
- S. Chavda and P. Bahadur, Micellization of a cationic gemini surfactant in aqueous solutions with different alkanols and alkanediols as additives: effect of nonpolar chain and position of hydroxyl groups, J. Mol. Liq., 2011, 161, 72–77 CrossRef CAS.
- N. Dharaiya, P. Bahadur, K. Singh, D. G. Marangoni and P. Bahadur, Light scattering and NMR studies of Triton X-100 micelles in the presence of short chain alcohols and ethoxylates, Colloids Surf., A, 2013, 436, 252–259 CrossRef CAS.
- A. Parmar, B. Bharatiya, K. Patel, V. Aswal and P. Bahadur, Alkanol-induced micelles of a very hydrophilic EO–PO–EO block copolymer: characterization by spectral and scattering methods, J. Surfactants Deterg., 2013, 16, 105–114 CrossRef CAS.
- P. Parekh, K. Singh, D. Marangoni and P. Bahadur, Effect of alcohols on aqueous micellar solutions of PEO–PPO–PEO copolymers: a dynamic light scattering and 1H NMR study, J. Mol. Liq., 2012, 165, 49–54 CrossRef CAS.
- V. Patel, J. Dey, R. Ganguly, S. Kumar, S. Nath, V. Aswal and P. Bahadur, Solubilization of hydrophobic alcohols in aqueous pluronic solutions: investigating the role of dehydration of the micellar core in tuning the restructuring and growth of pluronic micelles, Soft Matter, 2013, 9, 7583–7591 RSC.
- B. Bharatiya, C. Guo, J. Ma, P. Hassan and P. Bahadur, Aggregation and clouding behavior of aqueous solution of EO–PO block copolymer in presence of n-alkanols, Eur. Polym. J., 2007, 43, 1883–1891 CrossRef CAS.
- R. Oda, L. Bourdieu and M. Schmutz, Micelle to vesicle transition induced by cosurfactant: rheological study and direct observations, J. Phys. Chem. B, 1997, 101, 5913–5916 CrossRef CAS.
- A. Stradner, O. Glatter and P. Schurtenberger, A hexanol-induced sphere-to-flexible cylinder transition in aqueous alkyl polyglucoside solutions, Langmuir, 2000, 16, 5354–5364 CrossRef CAS.
- S. Candau and R. Zana, Effect of alcohols on the properties of micellar systems: III. Elastic and quasielastic light scattering study, J. Colloid Interface Sci., 1981, 84, 206–219 CrossRef CAS.
- E. Hirsch, S. Candau and R. Zana, Micellar structure and intermicellar interactions in solutions of tetradecyltrimethylammonium bromide in the presence of 1-pentanol: light scattering and viscosity study, J. Colloid Interface Sci., 1984, 97, 318–326 CrossRef CAS.
- H. Hoffmann, C. Thunig and M. Valiente, The different phases and their macroscopic properties in ternary surfactant systems of alkyldimethylamine oxides, intermediate chain n-alcohols and water, Colloids Surf., 1992, 67, 223–237 CrossRef CAS.
- H. Hoffmann, U. Munkert, C. Thunig and M. Valiente, The lyotropic mesophases in dilute surfactant mixtures of tetradecyldimethylaminoxide, tetradecyltrimethylammonium bromide, and hexanol: the influence of ionic charge on the mesophases, J. Colloid Interface Sci., 1994, 163, 217–228 CrossRef CAS.
- M. Gradzielski, M. Bergmeier, M. Müller and H. Hoffmann, Novel gel phase: a cubic phase of densely packed monodisperse, unilamellar vesicles, J. Phys. Chem. B, 1997, 101, 1719–1722 CrossRef CAS.
- L. Sreejith, S. Parathakkat, S. M. Nair, S. Kumar, G. Varma, P. A. Hassan and Y. Talmon, Octanol-triggered self-assemblies of the CTAB/KBr system: a microstructural study, J. Phys. Chem. B, 2010, 115, 464–470 CrossRef PubMed.
- X. Guo, H. Li, F. Zhang, S. Zheng and R. Guo, Aggregation of single-chained cationic surfactant molecules into vesicles induced by oligonucleotide, J. Colloid Interface Sci., 2008, 324, 185–191 CrossRef CAS PubMed.
- H. Akatsuka, M. Yamamoto, Y. Ohara and Y. Otsubo, Effect of polyols on the shear-induced structure and rheological properties of behenyl trimethyl ammonium chloride/1-octadecanol/water ternary systems, Colloids Surf., A, 2008, 326, 169–174 CrossRef CAS.
- E. W. Kaler, A. K. Murthy, B. E. Rodriguez and J. Zasadzinski, Spontaneous vesicle formation in aqueous mixtures of single-tailed surfactants, Science, 1989, 245, 1371–1374 CAS.
- E. W. Kaler, K. L. Herrington, A. K. Murthy and J. A. Zasadzinski, Phase behavior and structures of mixtures of anionic and cationic surfactants, J. Phys. Chem., 1992, 96, 6698–6707 CrossRef CAS.
- V. Patel, B. Bharatiya, D. Ray, V. Aswal and P. Bahadur, Investigations on microstructural changes in pH responsive mixed micelles of Triton X-100 and bile salt, J. Colloid Interface Sci., 2015, 441, 106–112 CrossRef CAS PubMed.
- D. Danino, A. Bernheim-Groswasser and Y. Talmon, Digital cryogenic transmission electron microscopy: an advanced tool for direct imaging of complex fluids, Colloids Surf., A, 2001, 183, 113–122 CrossRef.
- L. Abezgauz, K. Kuperkar, P. A. Hassan, O. Ramon, P. Bahadur and D. Danino, Effect of hofmeister anions on micellization and micellar growth of the surfactant cetylpyridinium chloride, J. Colloid Interface Sci., 2010, 342, 83–92 CrossRef CAS PubMed.
- D. Danino, Cryo-TEM of soft molecular assemblies, Curr. Opin. Colloid Interface Sci., 2012, 17, 316–329 CrossRef CAS.
- S. Shah, M. Ali Awan, M. Ashraf and S. Idris, Solubilization of phenol and benzyl alcohol by cationic and anionic surfactant micelles, Colloids Surf., A, 1995, 105, 319–323 CrossRef CAS.
- J. W. Larsen and L. B. Tepley, Effect of aqueous alcoholic solvents on counterion-binding to CTAB micelles, J. Colloid Interface Sci., 1974, 49, 113–118 CrossRef CAS.
- W.-Y. Yu, Y.-M. Yang and C.-H. Chang, Cosolvent effects on the spontaneous formation of vesicles from 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 anionic and cationic surfactant mixtures, Langmuir, 2005, 21, 6185–6193 CrossRef CAS PubMed.
1 anionic and cationic surfactant mixtures, Langmuir, 2005, 21, 6185–6193 CrossRef CAS PubMed. - V. Agarwal, M. Singh, G. McPherson, V. John and A. Bose, Microstructure evolution in aqueous solutions of cetyl trimethylammonium bromide (CTAB) and phenol derivatives, Colloids Surf., A, 2006, 281, 246–253 CrossRef CAS.
- W.-C. Zhang, G.-Z. Li, Q. Shen and J.-H. Mu, Effect of benzyl alcohol on the rheological properties of CTAB/KBr micellar systems, Colloids Surf., A, 2000, 170, 59–64 CrossRef CAS.
- K. Aramaki, S. Hoshida and S. Arima, Effect of carbon chain length of cosurfactant on the rheological properties of nonionic wormlike micellar solutions formed by a sugar surfactant and monohydroxy alcohols, Colloids Surf., A, 2010, 366, 58–62 CrossRef CAS.
- Y. I. González and E. W. Kaler, Cryo-TEM studies of worm-like micellar solutions, Curr. Opin. Colloid Interface Sci., 2005, 10, 256–260 CrossRef.
- V. R. Chaudhari, S. K. Haram, S. K. Kulshreshtha, J. R. Bellare and P. A. Hassan, Micelle assisted morphological evolution of silver nanoparticles, Colloids Surf., A, 2007, 301, 475–480 CrossRef CAS.
- A. Bernheim-Groswasser, R. Zana and Y. Talmon, Sphere-to-cylinder transition in aqueous micellar solution of a dimeric (gemini) surfactant, J. Phys. Chem. B, 2000, 104, 4005–4009 CrossRef CAS.
- K. Kuperkar, L. Abezgauz, D. Danino, G. Verma, P. Hassan, V. Aswal, D. Varade and P. Bahadur, Structural investigation of viscoelastic micellar water/CTAB/NaNO3 solutions, Pramana, 2008, 71, 1003–1008 CrossRef CAS.
- A. Khatory, F. Kern, F. Lequeux, J. Appell, G. Porte, N. Morie, A. Ott and W. Urbach, Entangled versus multiconnected network of wormlike micelles, Langmuir, 1993, 9, 933–939 CrossRef CAS.
- V. Croce, T. Cosgrove, C. A. Dreiss, S. King, G. Maitland and T. Hughes, Giant micellar worms under shear: a rheological study using SANS, Langmuir, 2005, 21, 6762–6768 CrossRef CAS PubMed.
- R. Kumar, G. C. Kalur, L. Ziserman, D. Danino and S. R. Raghavan, Wormlike micelles af a C22-tailed zwitterionic betaine surfactant: from viscoelastic solutions to elastic gels, Langmuir, 2007, 23, 12849–12856 CrossRef CAS PubMed.
- T. S. Davies, A. M. Ketner and S. R. Raghavan, Self-assembly of surfactant vesicles that transform into viscoelastic wormlike micelles upon heating, J. Am. Chem. Soc., 2006, 128, 6669–6675 CrossRef CAS PubMed.
- V. Patel, N. Dharaiya, D. Ray, V. K. Aswal and P. Bahadur, pH controlled size/shape in CTAB micelles with solubilized polar additives: a viscometry, scattering and spectral evaluation, Colloids Surf., A, 2014, 455, 67–75 CrossRef CAS.
- J.-h. Ma, C. Guo, Y.-l. Tang and H.-z. Liu, 1H NMR spectroscopic investigations on the micellization and gelation of PEO–PPO–PEO block copolymers in aqueous solutions, Langmuir, 2007, 23, 9596–9605 CrossRef CAS PubMed.
- V. Patel, D. Ray, V. K. Aswal and P. Bahadur, Triton X-100 micelles modulated by solubilized cinnamic acid analogues: the pH dependant micellar growth, Colloids Surf., A, 2014, 450, 106–114 CrossRef CAS.
- M. K. Mullally, M. J. Doyle and D. G. Marangoni, The partitioning of alkanediols into SDS and DTAB micelles from NMR-PRE experiments, Colloid Polym. Sci., 2004, 283, 335–339 CAS.
- M. Shi-Zhen and D. You-Ru, 1H NMR studies of surfactants in aqueous solutions, Acta Phys.-Chim. Sin., 2003, 7, 675–680 Search PubMed.
- B. E. Hawrylak and D. G. Marangoni, A 2D NMR investigation of the micellar solubilization site in ionic micellar solutions, Can. J. Chem., 1999, 77, 1241–1244 CAS.
- S. Macura and R. Ernst, Elucidation of cross relaxation in liquids by two-dimensional NMR spectroscopy, Mol. Phys., 1980, 41, 95–117 CrossRef CAS.
- L. Ziserman, L. Abezgauz, O. Ramon, S. R. Raghavan and D. Danino, Origins of the viscosity peak in wormlike micellar solutions. 1. Mixed catanionic surfactants. A cryo-transmission electron microscopy study, Langmuir, 2009, 25, 10483–10489 CrossRef CAS PubMed.
- T. Imae and T. Kohsaka, Size and electrophoretic mobility of tetradecyltrimethylammonium salicylate (C14TASal) micelles in aqueous media, J. Phys. Chem., 1992, 96, 10030–10035 CrossRef CAS.
- J. Pang, J. E. Hampsey, Q. Hu, Z. Wu, V. T. John and Y. Lu, Mesoporous silica with Ia3D cubic structure and good thermal stability, Chem. Commun., 2004, 6, 682–683 RSC.
| This journal is © The Royal Society of Chemistry 2015 |