A nitrogen and sulfur co-doped graphene-supported nickel tetrapyridyloxyphthalocyanine hybrid fabricated by a solvothermal method and its application for the detection of bisphenol A†
Bowen Zhang,
Yishu Wang,
Xvhong Dai,
Dajun Liu and
Xingquan He*
Department of Chemistry and Chemical Engineering, Changchun University of Science and Technology, Changchun 130022, P. R. China. E-mail: hexingquan@hotmail.com; Tel: +86-431-85583430
First published on 29th September 2015
Abstract
A novel hybrid consisting of nitrogen/sulfur co-doped graphene (N-S-G) and nickel tetrapyridyloxyphthalocyanine (NiTPPc) was fabricated by a solvothermal method and used for the voltammetric determination of bisphenol A (BPA). Compared with either pristine NiTPPc or N-S-G, the composite exhibits superior electrocatalytic activity towards the oxidation of BPA. Its oxidation peak current shows a linear dependence over the BPA concentration in the range from 0.2 to 122 μM, with a detection limit of 25.5 nM (S/N = 3). Furthermore, the fabricated NiTPPc/N-S-G sensor has been applied for the determination of BPA in commercial plastic samples.
1. Introduction
Bisphenol A (BPA), one of the key raw materials widely used to manufacture polycarbonate plastics, epoxy resins and polyacrylates, is known as an endocrine disrupting compound (EDCs) that is released into the environment from landfill leachates, milk bottles and plastics plants.1–3 Some studies suggest that BPA also exhibits an adverse effect in human immune function, and a correlation was found between BPA and diabetes, heart diseases and elevation of the levels of some serum liver enzymes.4,5 At present, various methods have already been proposed to detect BPA, such as fluorimetry,6 enzyme-linked immunosorbent assay,7 flow injection chemiluminescence,8 and electrochemical methods.9–11 Among these strategies, electrochemical methods have attracted much attention because of low cost of instrumentation, high sensitivity, simplicity for operators and portability. Various materials have been utilized to construct and modify the electrodes for detect BPA, such as conductive polymers,12 metal nanoparticles,9,13 MN4-centers of transition metal N4-macrocycles,14,15 and carbon based nanomaterials.16,17Metal phthalocyanines (MPcs) with highly conjugated π-electron system, macrocyclic hollow channel, considerable specific surface area and prominent electromagnetic properties, have received great attention.18 The MPcs modified electrodes have been used to the determination of some biologically and environmentally important molecules, such as nitrite,19 hydrazine,20 dopamine21 and BPA.14,15
Recently, much attention has been paid to the doping of heteroatoms into carbon matrices to modify the chemical and physical properties of the carbon materials.22–24 In particular, nitrogen- and sulfur-doped graphene materials have attracted much attention. It is generally believed that graphitic-N and pyridinic-N improve the catalytic activity of graphene in N-doped graphene (N-G), and thiophene-S plays a decisive role in sulfur-doped graphene (S-G).25,26 These nitrogen- and sulfur-doped graphene materials have been applied in many fields including batteries,27,28 electrocatalysis,29,30 and electrochemical biosensors.26,31 To further improve the performance of N-G and S-G, the nitrogen and sulfur co-doped graphene (N-S-G) has been researched as well.32–34
Inspired by excellent electrocatalytic performance of MPcs or doped graphene towards the detection of BPA,14,15,35,36 we think the MPcs/N-S-G composite modified GCE could further improve the detection limit and sensitivity of the fabricated BPA sensor due to the synergistic effect between MPcs and N-S-G.
Herein, a novel hybrid consisting of nickel tetrapyridyloxyphthalocyanine (NiTPPc) and N-S-G, abbreviated as NiTPPc/N-S-G, was obtained via a solvothermal method and applied for the determination of BPA. Compared with N-S-G or NiTPPc, the NiTPPc/N-S-G hybrid significantly improved the current response of the determination of BPA and greatly reduced overpotentials for the oxidation of BPA. Under optimum conditions, the linear calibration plot for BPA was obtained within the range of 0.2 to 122 μM and the detection limit was 25.5 nM (S/N = 3).
2. Experimental
2.1 Materials
Graphite powder was purchased from Sinopharm Chemical Reagent Co., Ltd. 4-Nitrophthalonitrile and 4-hydroxypyridine were bought from Sinopharm Chemical Reagent Co., Ltd. Pt/C (20 wt% Pt on Vulcan XC-72) was purchased from Alfa Aesar. 5-Amino-1,3,4-thiadiazole-2-thiol (ATT) was obtained from Sigma-Aldrich. Phosphate buffer solutions (PBS) at various pH values were prepared by mixing the stock solutions of 0.2 M NaH2PO4 and 0.2 M Na2HPO4. All other reagents were analytical grade and used without further purification, including N,N-dimethylformamide (DMF), ethanol, NaNO3, KMnO4 and H2O2. Ultra pure water was obtained from a Milli-Q water system (18.2 MΩ cm).2.2 Preparation of NiTPPc/N-S-G composite
Nickel tetrapyridyloxyphthalocyanine (NiTPPc) was synthesized and purified according to the method described in literature.37 Graphite oxide (GO) was prepared from natural flaked graphite using a modified Hummers method.38 The composite of NiTPPc and nitrogen/sulfur co-doped graphene (N-S-G), denoted as NiTPPc/N-S-G, was fabricated by the following procedure: 0.09 g graphene oxide (9 mL dispersion solution in DMF), 0.03 g NiTPPc and 0.18 g ATT were dispersed in 15 mL DMF under ultrasonic vibration for 30 min. Following, the dispersion solution was sealed in a 50 mL Teflon-lined autoclave and maintained at 160 °C for 12 h. Afterwards, its temperature was naturally cooled to the room degree to allow for the powder separation. The product was then centrifuged and washed with DMF, water and ethanol for several times. Simultaneously, the N-S-G was also prepared through the same steps as those used to make NiTPPc/N-S-G composite without the addition of 0.03 g NiTPPc in the ultrasonic vibration step.2.3 Characterization
Morphologies and structure of the NiTPPc/N-S-G composites were studied by transmission electron microscopy (TEM, JEOL JEM-2100F, operating at 200 kV) and X-ray diffraction studies (BRUKER, D8 ADVANCE, Germany). The UV-vis spectra were operated on a mini UV-1240 spectrophotometer. Fourier transform infrared spectroscopy measurement was performed using a FTIR-8400S spectrometer (KBr pellets). And analysis of the X-ray photoelectron spectra (XPS) was performed on an ESCLAB 250 spectrometer using Al Kα as the exciting source (1486.6 eV photons).2.4 Electrode preparation and electrochemical measurements
The glassy carbon electrode (GCE, 3.0 mm in diameter) was polished carefully using 1.0, 0.3, 0.05 μm alumina slurries successively, ultrasonically rinsed thoroughly with ethanol and water for one minute, and then blow-dried with nitrogen. The NiTPPc/N-S-G was dispersed into ethyl alcohol under ultrasonication to form a homogeneous ink at a concentration of 1.5 mg mL−1. A certain amount of the catalyst ink was dropped onto a freshly polished electrode surface to prepare the catalyst film. The catalyst loading per area on the GCE was kept to be 277 μg cm−2. For comparison, the NiTPPc or N-S-G modified GCE was also prepared using the same procedure and catalyst loading.Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements were performed on a CHI660D electrochemical workstation (CH Instruments, USA) in a conventional three-electrode cell using the coated GCE as the working electrode, a platinum wire as the auxiliary electrode, and a saturated calomel electrode (SCE) as reference. The CV and DPV measurements were performed in 20 mL PBS (pH = 7.0). The electrode was cyclically scanned in the range 0 to 1.0 V at a scan rate of 50 mV s−1.
3. Results and discussion
3.1 Characterizations of NiTPPc/N-S-G composite
Fig. 1 shows the transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images of the N-S-G and NiTPPc/N-S-G. The TEM image discloses that the N-S-G presents typical crumpled nanosheets structure (Fig. 1A). The HRTEM image reveals that the average interlayer distance of N-S-G is around 0.35 nm (Fig. 1B). Compared with the graphite interspacing of 0.34 nm, the enlarged interspacing indicates a moderate oxidation level presents in N-S-G (Fig. 1B). In Fig. 1C, NiTPPc nanocrystals with an average size of around 10 nm can be clearly seen. Representative HRTEM image confirms that the NiTPPc nanocrystals have a crystalline structure (Fig. 1D). The TEM image of NiTPPc/N-S-G displays that NiTPPc nanocrystals are uniformly loaded on the surface of N-S-G nanosheets, suggesting the growth of NiTPPc nanocrystals on N-S-G under the solvothermal conditions (Fig. 1E). The HRTEM image confirms that the mean size of NiTPPc is close to 10 nm (Fig. 1F), which is in agreement with the result of Fig. 1C. | ||
| Fig. 1 TEM images of (A) N-S-G, (C) NiTPPc, (E) NiTPPc/N-S-G, HRTEM images of (B) N-S-G, (D) NiTPPc, (F) NiTPPc/N-S-G. The inset shows TEM images of NiTPPc and NiTPPc/N-S-G. | ||
X-ray diffraction (XRD) was carried out to further study the crystallographic structure of the NiTPPc and NiTPPc/N-S-G (Fig. S1†). X-ray diffraction (XRD) was carried out to further study the crystallographic structure of the NiTPPc and NiTPPc/N-S-G (Fig. S1†). X-ray diffraction (XRD) was carried out to further study the crystallographic structure of the NiTPPc and NiTPPc/N-S-G (Fig. S1†). For NiTPPc, a broad peak at 2θ = 26° can be clearly seen, which is assigned to (002) diffraction peak of NiTPPc.39 The diffraction peak corresponding to the (110) plane of the Ni–Nx phase can also be found at 2θ = ∼33°, which should be ascribed to structure characteristic of the metal phthalocyanine.39 For the NiTPPc/N-S-G hybrid, the strong peak at 25.5° is contributed to the peak of NiTPPc at 2θ = 26° and the peak of graphene at 2θ = 25.3°.40 And the peak at 2θ = ∼33° is assigned to NiTPPc. In addition, the diffraction peak at 2θ = ∼43° is the (101) diffraction peak of graphite,40 another typical diffraction peak of graphene.
In an effort to better explore the π–π interactions between NiTPPc and N-S-G, FTIR, UV-vis and XPS spectra were utilized. As shown in Fig. S2,† the representative peaks at 1607, 1474, 1399, 1256, 1185, 1141, and 1098 cm−1 in the FTIR spectrum of the NiTPPc/N-S-G composite can be assigned to NiTPPc, indicating that the NiTPPc is clearly present in the hybrid.41 Fig. 2 elucidates the UV-vis spectra of N-S-G (black line), NiTPPc (green line) and NiTPPc/N-S-G (red line) in DMF, respectively. It can be seen that N-S-G shows a moderate absorption band at 273 nm due to the characteristic π-plasmon absorption42 and no absorption peaks can be found between 500 and 900 nm. To NiTPPc, there are two peaks of B band at 268 (the shoulder peak) and 308 nm, and two peaks of Q band at 627 and 677 nm, respectively.43,44 Meanwhile, the NiTPPc/N-S-G composite exhibits two peaks of B band at 278 and 312 nm, and two peaks of Q band (at 630 and 686 nm) with red-shift of 3 and 9 nm, which correspond to the characteristic absorption peaks of NiTPPc. Above results further confirm presence of the phthalocyanine in the sample.45
Fig. S3† shows the UV-vis spectra of NiTPPc in the absence (curve a) and presence (curve b) of BPA recorded in DMF. For the NiTPPc with BPA, the Q band (λmax = 631 nm) leads to a bathochromic shift of 5 nm compared with that for the NiTPPc without BPA (λmax = 626 nm), which should be related to axial ligation of BPA to NiTPPc.46
The interactions between NiTPPc and N-S-G are further confirmed by XPS. Fig. S4† presents the XPS survey spectra of NiTPPc, N-S-G and NiTPPc/N-S-G, respectively. For N-S-G, the peaks at around 398.0 and 164.0 eV refer to the nitrogen and sulfur atoms, respectively, demonstrating successful incorporation of N and S into graphene under the solvothermal conditions. For NiTPPc/N-S-G, Ni2p, N1s, O1s and S2p signals can be clearly observed. In addition, compared with NiTPPc and N-S-G, the relative intensities of O1s and N1s signals apparently increase while the relative intensity of S2p signal is nearly invariable in NiTPPc/N-S-G, indicating that NiTPPc was successfully loaded on N-S-G.
As depicted in Fig. 3A, the Ni2p3/2 peak is centered at 855.26 eV and the Ni2p1/2 peak is located at 873.07 eV in NiTPPc (Fig. 3A). Compared with NiTPPc, the Ni2p3/2 and Ni2p1/2 values of the NiTPPc/N-S-G composite shift to 855.78 and 873.14 eV, respectively (Fig. 3B), suggesting the interactions between NiTPPc and N-S-G lead to a decrease in the electron density on the Ni atom.47
The high-resolution S2p spectra of N-S-G and NiTPPc/N-S-G are shown in Fig. 3C and D respectively. N-S-G can be resolved into three different peaks (Fig. 3C), the lower binding energy signals correspond to the spin–orbit coupling positions of 2p3/2 (162.2 eV) and 2p1/2 (164.05 eV) for thiophene-S, the higher binding energy signal to SOx (168.54 eV) moieties.48 Compared with N-S-G, for NiTPPc/N-S-G, the peaks of the thiophene-S (2p3/2) (162.34 eV) and SOx-like moieties (168.74 eV) shift to higher binding energies by 0.14 eV and 0.2 eV, respectively (Fig. 3D).
From deconvolution of the N1s XPS spectrum (Fig. 3E), the spectrum for N-S-G could be well-fitted to three peaks with binding energies at 398.86 eV (pyridinic type nitrogen), 399.67 eV (pyrrolic type nitrogen), and 400.85 eV (graphitic type nitrogen).49 However, the corresponding N1s peaks for NiTPPc/N-S-G shift to higher binding energies by 0.14 eV, 0.1 eV and 0.17 eV compared with those for pure N-S-G, respectively, as shown in Fig. 3F. Such a slight shift of Ni2p, S2p or N1s in binding energies demonstrates the electronic interactions between NiTPPc moieties and N-S-G sheets.
3.2 Characterization of the modified electrodes
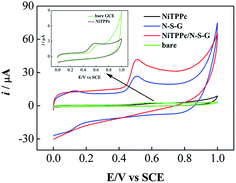
The electrocatalytic activity of the modified GCEs towards the oxidation of BPA was first examined by CV. Fig. 4 shows the CVs of bare GCE (green line), NiTPPc (black line), N-S-G (blue line) and NiTPPc/N-S-G (red line) modified GCEs at the scan rate of 50 mV s−1 in PBS (pH = 7.0) containing 0.1 mmol L−1 of BPA. The inset presents the enlarged CVs of bare GCE and NiTPPc. As can be seen from the inset in Fig. 4, the bare GCE or NiTPPc modified GCE displays a poor current response for the oxidation of BPA. At the N-S-G modified GCE, the peak current obviously increases for the oxidation of BPA and the oxidation peak potential of BPA shifts about 130 mV to the negative side compared with that at the bare GCE, suggesting a fast electron transfer rate for the oxidation of BPA. In contrast, the peak current of BPA oxidation on the NiTPPc/N-S-G modified GCE is about 1.6 times than that on N-S-G modified GCE and the peak potential of BPA oxidation on the NiTPPc/N-S-G barely changes compared with that on N-S-G, demonstrating the excellent electrocatalytic activity of the NiTPPc/N-S-G hybrid. The enhanced catalytic activity of the NiTPPc/N-S-G hybrid should be due to the synergistic effect between NiTPPc and N-S-G.The electrochemically effective surface area of the modified GCE was estimated by cyclic voltammetry using K3[Fe(CN)6] as a probe and according to the Randles–Sevcik equation:50
| ip = 2.69 × 105nAC0D1/2v1/2 | (1) |
3.3 Effect of pH on the oxidation of BPA
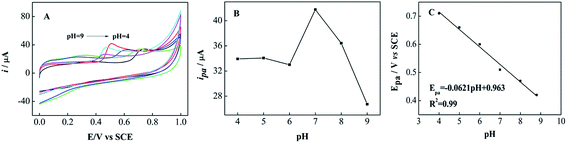
The influence of pH values on the peak potentials and peak currents of the NiTPPc/N-S-G modified electrode for BPA oxidation was also studied by CV in the pH range from 4 to 9. As seen from Fig. 6A, anodic peak potentials shift negatively with the increase of pH, indicating that the oxidation of BPA includes some protons transfer at the NiTPPc/N-S-G modified electrode. The oxidation current versus pH varying the range from 4.0 to 9.0 can be clearly seen from Fig. 6B. As our results showed, the maximum anodic current of BPA is obtained at pH 7.0. Therefore, the pH 7.0 was selected for all electrochemical measurements in this study. A well linear relationship between the anodic peak potential (Epa) and pH is obtained with the equation Epa (V) = −0.0621pH + 0.963 (R2 = 0.99) (Fig. 6C). The slope of −62.1 mV per pH is close to the theoretical value (−59 mV per pH), which indicates that the equal number of protons and electrons are involved in the electrochemical redox process of BPA. This is consistent with the previous report.513.4 Effect of scan rate on the peak current of BPA
Fig. 7A shows the CVs of NiTPPc/N-S-G composite film modified GCE at various scan rates in PBS (pH = 7.0) containing 0.1 mmol L−1 BPA respectively. With the increase of scan rate, the anodic peak currents increase apparently, and the anodic peak potential shifts positively. As illustrated in Fig. 7B, the linear regression equation is ipa (μA) = 0.41v (mV s−1) + 9.76 with correlation coefficients of R2 = 0.996, respectively, suggesting that the electrochemical reaction is a typical adsorption-controlled process. A linear relationship between Epa and Napierian logarithm of v (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) is also observed in the range of 30–300 mV s−1 (Fig. S5†) and the equation can be expressed as Epa (V) = 0.4018 + 0.030
v) is also observed in the range of 30–300 mV s−1 (Fig. S5†) and the equation can be expressed as Epa (V) = 0.4018 + 0.030![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v (mV s−1) (R2 = 0.996). As for an electron transfer controlled and totally irreversible electrode process, Epa is defined by the following equation:
v (mV s−1) (R2 = 0.996). As for an electron transfer controlled and totally irreversible electrode process, Epa is defined by the following equation:where α is transfer coefficient, k0 is standard rate constant of the reaction, n is electron transfer number involved in rate-determining step, v is scanning rate, E0 is formal redox potential, R is the gas constant, T is the absolute temperature, and F is the faraday constant. According to the linear correlation of Epa vs. ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v as mentioned above, the slope of the line is equal to RT/αnF, therefore αn can be easily calculated from the corresponding slope of Epa–ln
v as mentioned above, the slope of the line is equal to RT/αnF, therefore αn can be easily calculated from the corresponding slope of Epa–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v. In this work, the slope of the line is 0.030. Therefore, the value of αn is calculated to be 0.86 (taking T = 298 K, R = 8.314 J K−1 mol−1, and F = 96
v. In this work, the slope of the line is 0.030. Therefore, the value of αn is calculated to be 0.86 (taking T = 298 K, R = 8.314 J K−1 mol−1, and F = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1). Generally, α is assumed to be 0.5 in totally irreversible electrode process, so the electron transfer number (n) is around 2 for the electrochemical oxidation of BPA. Considering that the number of electrons and protons involved in the BPA oxidation process is equal,52 the oxidation of BPA on the NiTPPc/N-S-G is a two-electron and two-proton process.
485 C mol−1). Generally, α is assumed to be 0.5 in totally irreversible electrode process, so the electron transfer number (n) is around 2 for the electrochemical oxidation of BPA. Considering that the number of electrons and protons involved in the BPA oxidation process is equal,52 the oxidation of BPA on the NiTPPc/N-S-G is a two-electron and two-proton process.
In the light of our results and precious reports of electrochemical reaction mechanism of phenolic compounds,53–55 the mechanism for the BPA oxidation on the NiTPPc/N-S-G modified GCE can be reasonably deduced as follows (Scheme 1):
We speculate that the following two-step mechanism is responsible for the electrocatalytic oxidation of BPA on Ni(II)TPPc. Firstly, BPA interacts with Ni(III)TPPc to form an axial coordination complex (eqn (1)).14,56 Then Ni(II)TPPc is electrochemically oxidized to produce Ni(III)TPPc before the oxidation of BPA (eqn (2)) because NiIII/NiII redox potential (0.2–0.5 V) (Fig. S6†) is lower than the oxidation potential of BPA. Finally, the complex loses two H+ to generate a quinoid substance and Ni(II)TPPc (eqn (3)).
3.5 Determination of BPA
DPV is a more sensitive technique that has been widely used for electrochemical detections.57 Usually, in order to stabilize the modified electrode before the test, the modified electrode was adjusted by potential scanning repeatedly in a blank PBS of pH = 7.0 at least 10 segments. Fig. 8A shows DPV curves of different concentrations of BPA on NiTPPc/N-S-G electrode. The detection range of BPA is from 0.2 to 122 μM (Fig. 8B) and the linear relationship between peak current and BPA concentration is shown in Fig. 8B. The linear regression equation is expressed as ipa (μA) = 0.093C (μM) + 4.82, with a correlation coefficient R2 = 0.987. The detection limit is obtained to be 25.5 nM at a signal to noise ratio of 3 and the sensitivity of the sensor is 93 μA mM−1. For comparison, we studied the performance of the NiTPPc modified GCE and N-S-G modified GCE for the determination of BPA (Fig. S7 and S8†). The summarized results are shown in ESI Table S1.† The performance of our fabricated BPA sensor is compared with those previously reported in literatures,10–13,16,17,58–60 as shown in Table 1. It is worthy to note that the detection limit and linear range of our proposed sensor is comparable to or even better than those of previous reported BPA sensors.| Electrode | Method | Linear range (μM) | Detection limit (μM) | Reference |
|---|---|---|---|---|
| MWCNT–MAM/GCE | IT | 0.01–40.8 | 0.005 | 10 |
| Graphene/GCE | DPV | 0.05–1 | 0.047 | 11 |
| MIP-NG/GCE | IT | 8–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.138 | 12 |
| Tyr/nano-Au/T-NH2/Au | CV | 0.399–234 | 0.133 | 13 |
| rGO-Fc-NH2/AuNPs/GCE | DPV | 0.005–10 | 0.002 | 16 |
| CS/MNPs-rGO/GCE | DPV | 0.06–11 | 0.017 | 17 |
| Tyr-SF-MWNTs-CoPc/GCE | IT | 0.05–3 | 0.03 | 58 |
| ctDNA-SWNT/GCE | DPV | 0.01–20 | 0.005 | 59 |
| f-SWCNT/PC4/GCE | CV | 0.099–5.794 | 0.03 | 60 |
| NiTPPc/N-S-G/GCE | DPV | 0.2–122 | 0.0255 | This work |
3.6 Determination of BPA in plastic containers
The utilization of the proposed method in real samples analysis was also investigated by direct analysis of BPA in three commercial plastic samples used for food packaging which were collected from local supermarkets. The mock samples (plastic extracts containing BPA and other contaminants) were prepared as described in the ESI.† The BPA concentration was determined using standard addition and the concentrations of BPA found with the respective recovery in B1, B2 and B3 are listed in Table 2. It can be summarized that the proposed electrochemical method for practical samples is accurate and reliable on the basis of the average recoveries varied from 96.33 to 98.67% with RSD below 5% in all cases.| Sample | Original (μmol L−1) | Added (μmol L−1) | Detected (μmol L−1) | RSD (%) | Recovery (%) |
|---|---|---|---|---|---|
| a n: test number of each sample. Original: concentration of BPA in the preliminary test liquid. Added: added concentration of BPA. RSD: relative standard deviation. Recovery: ratio of the detected concentration of BPA to the total concentration of original and added BPA. | |||||
| B1 | 4 | 8 | 11.56 | 1.43 | 96.33 |
| B2 | 4 | 8 | 11.58 | 1.56 | 96.50 |
| B3 | 4 | 8 | 11.84 | 1.24 | 98.67 |
4. Conclusions
In this paper, we have successfully fabricated a novel BPA sensor based on a NiTPPc/N-S-G hybrid modified GCE. The proposed sensor exhibits a remarkable electrocatalytic activity towards the oxidation of BPA and has been successfully used for the detection of BPA in plastic containers. Compared with NiTPPc modified GCE and N-S-G modified GCE, the NiTPPc/N-S-G modified GCE displays better electrocatalytic properties for the determination of BPA in terms of lower detection limit and higher sensitivity, which should be due to the synergistic effect between NiTPPc and N-S-G. All these results indicate that the NiTPPc/N-S-G has a bright future in analytical applications.Conflict of interest
The authors declare that they have no conflict of interest.Acknowledgements
This research has been financed by the National Natural Science Foundation of China (No. 21273024), Natural Science Foundation of Jilin Province, China (No. 201215135) and Scientific Research Foundation of the Education Department of Jilin Province, China (No. KYC-JC-XM-2013-021).References
- D. D. Pan, Y. Y. Gu, H. Z. Lan, Y. Y. Sun and H. J. Gao, Anal. Chim. Acta, 2015, 853, 297–302 CrossRef CAS PubMed.
- C. A. Staples, P. B. Dome, G. M. Klecka, S. T. Oblock and L. R. Harris, Chemosphere, 1998, 36, 2149–2173 CrossRef CAS.
- E. M. R. Clayton, M. Todd, J. B. Dowd and A. E. Aiello, Environ. Health Perspect., 2011, 119, 390 CrossRef CAS PubMed.
- P. H. Deng, Z. F. Xu and Y. F. Kuang, Food Chem., 2014, 157, 490–497 CrossRef CAS PubMed.
- Y. B. Wetherill, B. T. Akingbemi, J. Kannod, J. A. McLachlan, A. Nadal, C. Sonnenschein, C. S. Watson, R. T. Zoeller and S. M. Belcher, Reprod. Toxicol., 2007, 24, 178 CrossRef CAS PubMed.
- X. Wang, H. Zeng, L. Zhao and J. M. Lin, Anal. Chim. Acta, 2006, 556, 313–318 CrossRef CAS PubMed.
- R. Kuruto-Niwa, Y. Tateoka, Y. Usuki and R. Nozawa, Chemosphere, 2007, 66, 1160–1164 CrossRef CAS PubMed.
- S. Wang, X. Wei, L. Du and H. Zhuang, Luminescence, 2005, 20, 46–50 CrossRef CAS PubMed.
- L. L. Zhu, Y. H. Cao and G. Q. Cao, Biosens. Bioelectron., 2014, 54, 258–261 CrossRef CAS PubMed.
- Y. G. Li, Y. Gao, Y. Cao and H. M. Li, Sens. Actuators, B, 2012, 171–172, 726–733 CrossRef CAS PubMed.
- B. Ntsendwana, B. B. Mamba, S. Sampath and O. A. Arotiba, Int. J. Electrochem. Sci., 2012, 7, 3501–3512 CAS.
- J. D. Huang, X. M. Zhang, S. Liu, Q. Lin, X. R. He, X. R. Xing and W. J. Lian, J. Appl. Electrochem., 2011, 41, 1323–1328 CrossRef CAS.
- N. Wang, H. Y. Zhao, X. P. Ji, X. R. Li and B. B. Wang, Chin. Chem. Lett., 2014, 25, 720–722 CrossRef CAS PubMed.
- H. S. Yin, Y. L. Zhou and S. Y. Ai, J. Electroanal. Chem., 2009, 626, 80–88 CrossRef CAS PubMed.
- V. Chauke, F. Matemadombo and T. Nyokong, J. Hazard. Mater., 2010, 178, 180–186 CrossRef CAS PubMed.
- N. Huang, M. L. Liu, H. T. Li, Y. Y. Zhang and S. Z. Yao, Anal. Chim. Acta, 2015, 853, 249–257 CrossRef CAS PubMed.
- Y. X. Zhang, Y. X. Cheng, Y. Y. Zhou, B. Y. Li, W. Gu, X. H. Shi and Y. Z. Xian, Talanta, 2013, 107, 211–218 CrossRef CAS PubMed.
- J. H. Zagal, Coord. Chem. Rev., 1992, 119, 89–136 CrossRef CAS.
- W. J. R. Santos, A. L. Sousa, R. C. S. Luz, F. S. Damos, L. T. Kubota, A. A. Tanaka and S. M. C. N. Tanaka, Talanta, 2006, 70, 588–594 CrossRef CAS PubMed.
- E. F. Perez, G. O. Neto, A. A. Tanaka and L. T. Kubota, Electroanalysis, 1998, 10, 111–115 CrossRef CAS.
- F. C. Moraes, M. F. Cabral, S. A. S. Machado and L. H. Mascaro, Electroanalysis, 2008, 20, 851–857 CrossRef CAS PubMed.
- X. Wang, X. Li, L. Zhang, Y. Yoon, P. K. Weber, H. Wang, J. Guo and H. Dai, Science, 2009, 324, 768–771 CrossRef CAS PubMed.
- S. Yu, W. Zheng, C. Wang and Q. Jiang, ACS Nano, 2010, 4, 7619–7629 CrossRef CAS PubMed.
- Z. W. Liu, F. Peng, H. J. Wang, H. Yu, W. X. Zheng and J. Yang, Angew. Chem., 2011, 123, 3315–3319 CrossRef PubMed.
- Y. Wang, Y. Y. Shao, D. W. Matson, J. Li and Y. H. Lin, ACS Nano, 2010, 4, 1790–1798 CrossRef CAS PubMed.
- J. J. Shi, X. J. Zhou, P. Xu, J. L. Qiao, Z. W. Chen and Y. Y. Liu, Electrochim. Acta, 2014, 145, 259–269 CrossRef CAS PubMed.
- X. Wang, X. Q. Cao, L. Bourgeois, H. Guan, S. M. Chen, Y. T. Zhong, D. M. Tang, H. Q. Li, T. Y. Zhai, L. Li, Y. Bando and D. Golberg, Adv. Funct. Mater., 2012, 22, 2682–2690 CrossRef CAS PubMed.
- Y. Yan, Y. X. Yin, S. Xin, Y. G. Guo and L. J. Wan, Chem. Commun., 2012, 48, 10663–10665 RSC.
- L. T. Qu, Y. Liu, J. B. Baek and L. M. Dai, ACS Nano, 2010, 4, 1321–1326 CrossRef CAS PubMed.
- Z. Yang, Z. Yao, G. F. Li, G. Y. Fang, H. G. Nie, Z. Liu, X. M. Zhou, X. A. Chen and S. M. Huang, ACS Nano, 2012, 6, 205–211 CrossRef CAS PubMed.
- Y. X. Liu, Y. L. Ma, Y. Jin, G. Q. Chen and X. Zhang, J. Electroanal. Chem., 2015, 939, 172–177 CrossRef PubMed.
- W. Ai, Z. M. Luo, J. Jiang, J. H. Zhu, Z. Z. Du, Z. X. Fan, L. H. Xie, H. Zhang, W. Huang and T. Yu, Adv. Mater., 2014, 26, 6186–6192 CrossRef CAS PubMed.
- Q. Luo, F. Hao, S. H. Wang, H. P. Shen, L. H. Zhao, J. B. Li, M. Grätzel and H. Lin, J. Phys. Chem. C, 2014, 118, 17010–17018 CAS.
- G. Q. Chen, Y. X. Liu, Y. Liu, Y. Tian and X. Zhang, J. Electroanal. Chem., 2015, 738, 100–107 CrossRef CAS PubMed.
- S. F. Jiao, J. Jin and L. Wang, Talanta, 2014, 122, 140–144 CrossRef CAS PubMed.
- H. X. Fan, Y. Li, D. Wu, H. M. Ma, K. X. Mao, D. W. Fan, B. Du, H. Li and Q. Wei, Anal. Chim. Acta, 2012, 711, 24–28 CrossRef CAS PubMed.
- L. L. Cui, G. J. Lv and X. Q. He, J. Power Sources, 2015, 282, 9–18 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- R. Zhang, Y. X. Peng, Z. P. Li, K. Li, J. Ma, Y. Liao, L. R. Zheng, X. Zuo and D. G. Xi, Electrochim. Acta, 2014, 147, 343–351 CrossRef CAS PubMed.
- G. H. Yuan, J. T. Bai, T. N. L. Doan and P. Chen, Mater. Lett., 2015, 158, 248–251 CrossRef CAS PubMed.
- C. Z. Zhang, R. Hao, H. Yin, F. Liu and Y. L. Hou, Nanoscale, 2012, 4, 7326–7329 RSC.
- Z. W. Xu, G. X. Zhang, Z. Y. Cao, J. S. Zhao and H. J. Li, J. Mol. Catal. A: Chem., 2010, 318, 101–105 CrossRef CAS PubMed.
- İ. Değirmencioğlua, R. Bayraka, M. Erb and K. Serbestc, Dyes Pigm., 2009, 83, 51–58 CrossRef PubMed.
- A. Chunder, T. Pal, S. I. Khondaker and L. Zhai, J. Phys. Chem. C, 2010, 114, 15129–15135 CAS.
- G. Q. Mo, S. Y. Liao, Y. Z. Zhang, W. D. Zhang and J. S. Ye, Electrochim. Acta, 2012, 76, 430–439 CrossRef CAS PubMed.
- N. Grootboom and T. Nyokong, Anal. Chim. Acta, 2001, 432, 49–57 CrossRef CAS.
- J. Thompson, A. Crossley, P. D. Nellista and V. Nicolosi, J. Mater. Chem., 2012, 22, 23246–23253 RSC.
- B. X. Zhang, H. Gao and X. L. Li, New J. Chem., 2014, 38, 4615–4621 RSC.
- M. K. Liu, Y. F. Song, S. X. He, W. W. Tjiu, J. S. Pan, Y. Y. Xia and T. X. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 4214–4222 CAS.
- M. Jahan, Q. L. Bao and K. P. Loh, J. Am. Chem. Soc., 2012, 134, 6707–6713 CrossRef CAS PubMed.
- F. Y. Zhang, Y. J. Li, Y. E. Gu, Z. H. Wang and C. M. Wang, Microchim. Acta, 2011, 173, 103–109 CrossRef CAS.
- Y. Gao, Y. Cao and D. G. Yang, J. Hazard. Mater., 2012, 199–200, 111–118 CrossRef CAS PubMed.
- N. Grootboom and T. Nyokong, Anal. Chim. Acta, 2001, 432, 49–57 CrossRef CAS.
- M. S. Ureta-Zañartu, P. Bustos, C. Berríos, M. C. Diez, M. L. Mora and C. Gutiérrez, Electrochim. Acta, 2002, 47, 2399–2406 CrossRef.
- S. Andreescu, D. Andreescu and O. A. Sadik, Electrochem. Commun., 2003, 5, 681–688 CrossRef CAS.
- K. Y. Hou, L. Huang, Y. B. Qi, C. X. Huang, H. B. Pan and M. Du, Mater. Sci. Eng., B, 2015, 49, 640–647 CrossRef CAS PubMed.
- L. Y. Feng, X. Li, Y. H. Peng and J. Geng, Chem. Phys. Lett., 2009, 480, 309–312 CrossRef CAS PubMed.
- H. S. Yin, Y. L. Zhou, J. Xu, S. Y. Ai, L. Cui and L. S. Zhu, Anal. Chim. Acta, 2010, 659, 144–150 CrossRef CAS PubMed.
- X. H. Jiang, W. J. Ding, C. L. Luan, Q. Q. Ma and Z. Y. Guo, Microchim. Acta, 2013, 180, 1021–1028 CrossRef CAS.
- L. Zhang, Y. P. Wen, Y. Y. Yao, Z. F. Wang, X. M. Duan and J. K. Xu, Chin. Chem. Lett., 2014, 25, 517–522 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14516c |
| This journal is © The Royal Society of Chemistry 2015 |