Evaluation of a metal free dye for efficient dye sensitized solar cells based on hydrothermally synthesized TiO2 nanoflowers
Abstract
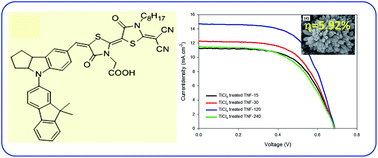
The main aim of the present investigation is to evaluate dye sensitized solar cells (DSSCs) properties of hydrothermally synthesized 3D TiO2 nanoflowers based on DN350 organic dye. The dye loading time has been optimized from the current–voltage (J–V) characteristics in order to obtain high performance DSSCs. Further TiCl4 surface treatment was conducted for effective dye loading and fast electron transport for the improvement of DSSC performance. The dye loading and its performance evaluation has been optimized and analysed using J–V performance, UV-vis spectroscopy and incident-photon-to-electron conversion efficiency (IPCE) etc. characterization techniques. The diffusion coefficient, diffusion length and electron life time have be evaluated using intensity-modulated photocurrent and photovoltage experiments (IMPS/IMVS). Our optimized results show that 30 min TiCl4 treated TNF samples exhibit current density (JSC) = 14.70 mA cm−2, open circuit voltage (VOC) = 0.650 V, Fill Factor (FF) = 0.62 and power conversion efficiency (PCE) = 5.92%.

 Please wait while we load your content...
Please wait while we load your content...